|
Acute chest syndrome
does not have a chronic inflammatory background
in sickle cell diseases
......................................................................................................................................................................
Mehmet Rami Helvaci (1)
Mustafa Sahan (2)
Nesrin Atci (3)
Orhan Ayyildiz (4)
Orhan Ekrem Muftuoglu (4)
Lesley Pocock (5)
(1) Medical Faculty of the Mustafa Kemal University,
Antakya, Professor of Internal Medicine, M.D.
(2) Medical Faculty of the Mustafa Kemal University,
Antakya, Assistant Professor of Radiology, M.D.
(3) Medical Faculty of the Dicle University, Diyarbakir,
Professor of Internal Medicine, M.D.
(4) medi+WORLD International, Australia
Correspondence:
Mehmet Rami Helvaci, M.D.
Medical Faculty of the Mustafa Kemal University,
31100, Serinyol, Antakya, Hatay, TURKEY
Phone: 00-90-326-2291000 (Internal 3399) Fax:
00-90-326-2455654
Email: mramihelvaci@hotmail.com
|
ABSTRACT
Background: Sickle cell diseases
(SCDs) are chronic catastrophic processes
on vascular endothelium initiating at birth
all over the body. We tried to understand
whether or not there is a chronic inflammatory
background of acute chest syndrome (ACS)
in the SCDs.
Methods: All patients with the SCDs
were taken into the study.
Results: The study included 411 patients
(199 females). As one of the significant
endpoints of SCDs, patients with chronic
obstructive pulmonary disease (COPD) and
without were collected into two groups.
There were 60 patients (14.5%) with COPD.
Mean age (33.0 versus 29.5 years, P=0.005)
and male ratio (80.0% versus 46.7%, P<0.001)
were higher in the COPD group. Smoking (36.6%
versus 9.9%, P<0.001) and alcohol (3.3%
versus 0.8%, P<0.05) were also higher
among the COPD cases. Transfused red blood
cell units in their lives (69.1 versus 32.9,
P=0.001), priapism (10.0% versus 1.9%, P<0.001),
leg ulcers (26.6% versus 11.6%, P<0.001),
digital clubbing (25.0% versus 7.1%, P<0.001),
coronary heart disease (26.6% versus 13.1%,
P<0.01), chronic renal disease (16.6%
versus 7.1%, P<0.01), and stroke (20.0%
versus 7.9%, P<0.001) were all higher
among the COPD cases, too. Interestingly,
against the higher rates of the above problems
in the COPD group, incidence of ACS was
even lower among them, nonsignificantly
(1.6% versus 3.9%, P>0.05).
Conclusion: SCDs cause severe chronic
endothelial damage particularly at the capillary
level, and terminate with accelerated atherosclerosis
induced end-organ failures in early years
of life. Probably ACS is a sudden onset
event without any chronic inflammatory background
in the SCDs.
Key words: Sickle cell diseases, acute
chest syndrome, chronic endothelial damage
|
Chronic endothelial damage may
be the major cause of aging by causing disseminated
tissue ischemia all over the body. For instance,
cardiac cirrhosis develops due to the prolonged
hepatic hypoxia in individuals with pulmonary
and/or cardiac diseases. Probably whole afferent
vasculature including capillaries are mainly involved
in the process. Some of the well-known accelerators
of the inflammatory process are physical inactivity,
weight gain, smoking, and alcohol for the development
of irreversible endpoints including obesity, hypertension
(HT), diabetes mellitus (DM), cirrhosis, peripheric
artery disease (PAD), chronic obstructive pulmonary
disease (COPD), chronic renal disease (CRD), coronary
heart disease (CHD), mesenteric ischemia, osteoporosis,
and stroke, all of which terminate with early
aging and death. They were researched under the
title of metabolic syndrome in the literature,
extensively (1, 2). Similarly, sickle cell diseases
(SCDs) are the causes of severe chronic endothelial
damage particularly at the capillary level. Hemoglobin
S (HbS) causes loss of elastic and biconcave disc
shaped structures of red blood cells (RBCs). Probably
loss of elasticity instead of shape is the major
problem since sickling is very rare in peripheric
blood samples of patients with associated thalassemia
minors, and human survival is not so affected
in hereditary spherocytosis or elliptocytosis.
Loss of elasticity is present in whole lifespan,
but exaggerated with stresses induced increased
metabolic rate of the body. The hard cells induce
prolonged endothelial inflammation, edema, and
fibrosis mainly at the capillary level and terminate
with disseminated cellular hypoxia all over the
body (3, 4). On the other hand, obvious vascular
occlusions may not develop in greater vasculature
due to their transport instead of distribution
function for the hard cells. We tried to understand
whether or not there is a chronic inflammatory
background of acute chest syndrome (ACS) in the
SCDs.
The study
was performed in the Medical Faculty of the Mustafa
Kemal University between March 2007 and July 2015.
All patients with SCDs were studied. The SCDs
are diagnosed with hemoglobin electrophoresis
performed via high performance liquid chromatography
(HPLC). Medical histories including smoking habit,
regular alcohol consumption, painful crises per
year, transfused RBC units in their lives, surgical
operations, priapism, leg ulcers, and stroke were
learnt. Patients with a history of one pack-year
were accepted as smokers, and one drink-year were
accepted as drinkers. Cases with acute painful
crisis or another inflammatory event were treated
at first, and the laboratory tests and clinical
measurements were performed on the silent phase.
A check up procedure including serum iron, iron
binding capacity, ferritin, creatinine, liver
function tests, markers of hepatitis viruses A,
B, and C and human immunodeficiency virus, a posterior-anterior
chest x-ray film, an electrocardiogram, a Doppler
echocardiogram both to evaluate cardiac walls
and valves and to measure the systolic blood pressure
(BP) of pulmonary artery, an abdominal ultrasonography,
a computed tomography of brain, and a magnetic
resonance imaging (MRI) of hips was performed.
Other bones for avascular necrosis were scanned
according to the patients' complaints. Associated
thalassemia minors were detected with serum iron,
iron binding capacity, ferritin, and hemoglobin
electrophoresis performed via HPLC. The criterion
for diagnosis of COPD is post-bronchodilator forced
expiratory volume in one second/forced vital capacity
of less than 70% (5). ACS is diagnosed clinically
with the presence of new infiltrates on chest
x-ray film, fever, cough, sputum production, dyspnea,
or hypoxia (6). An x-ray film of abdomen in upright
position was taken just in patients with abdominal
distention or discomfort, vomiting, obstipation,
or lack of bowel movement, and ileus was diagnosed
with gaseous distention of isolated segments of
bowel, vomiting, obstipation, cramps, and with
the absence of peristaltic activity on the abdomen.
Systolic BP of the pulmonary artery of 40 mmHg
or higher is accepted as pulmonary hypertension
(7). CRD is diagnosed with a persistent serum
creatinine level of 1.3 mg/dL in males and 1.2
mg/dL in females. Cirrhosis is diagnosed with
physical examination, hepatic function tests,
ultrasonographic results, and tissue sample in
case of indication. Digital clubbing is diagnosed
with the ratio of distal phalangeal diameter to
interphalangeal diameter which is greater than
1.0, and with the presence of Schamroth's sign
(8, 9). An exercise electrocardiogram is just
performed in cases with an abnormal electrocardiogram
and/or angina pectoris. Coronary angiography is
taken just for the exercise electrocardiogram
positive cases. So CHD was diagnosed either angiographically
or with the Doppler echocardiographic findings
as the movement disorders in the cardiac walls.
Rheumatic heart disease is diagnosed with the
echocardiographic findings, too. Avascular necrosis
of bones is diagnosed by means of MRI (10). Stroke
is diagnosed by the computed tomography of brain.
Ophthalmologic examination was performed according
to the patients' complaints. Eventually as one
of the significant endpoints of the SCDs, cases
with COPD and without were collected into the
two groups, and they were compared in between.
Mann-Whitney U test, Independent-Samples t test,
and comparison of proportions were used as the
methods of statistical analyses.
The study included 411 patients
with SCDs (199 females and 212 males). There were
60 patients (14.5%) with COPD. Mean age (33.0
versus 29.5 years, P=0.005) and male ratio (80.0%
versus 46.7%, P<0.001) were higher in the COPD
group. Smoking (36.6% versus 9.9%, P<0.001)
and alcohol (3.3% versus 0.8%, P<0.05) were
also higher among the COPD cases. Prevalence of
associated thalassemia minors were similar in
both groups (71.6% versus 66.6% in the COPD group
and other, respectively, P>0.05) (Table 1).
Beside these, transfused RBC units in their lives
(69.1 versus 32.9, P=0.001), priapism (10.0% versus
1.9%, P<0.001), leg ulcers (26.6% versus 11.6%,
P<0.001), digital clubbing (25.0% versus 7.1%,
P<0.001), CHD (26.6% versus 13.1%, P<0.01),
CRD (16.6% versus 7.1%, P<0.01), and stroke
(20.0% versus 7.9%, P<0.001) were all higher
among the COPD cases. Interestingly, against the
higher rates of above problems in the COPD group,
incidence of ACS was even lower among them, nonsignificantly
(1.6% versus 3.9%, P>0.05) (Table 2). The differences
according to the mean white blood cell (WBC) count,
hematocrit (Hct) value, and platelet (PLT) count
of peripheric blood were nonsignificant between
the two groups (Table 3). There were 27 mortalities
(14 males) during the nine-year follow up period,
and only two of them in the group without COPD
were due to the ACS. The mean ages of mortality
were 33.6 ± 9.5 years (range 19-47) in
females and 30.8 ± 8.9 years (range 19-50)
in males (P>0.05). On the other hand, there
were three patients with sickle cell retinopathy;
all of them were found in cases without COPD.
Additionally, there were four patients with HBsAg
positivity (0.9%) but HBV DNA was positive in
none of them by polymerase chain reaction (PCR).
Although antiHCV was positive in 6.0% (25) of
the study cases, HCV RNA was detected as positive
just in four (0.9%) by PCR.
Table 1: Characteristic features of the study
cases
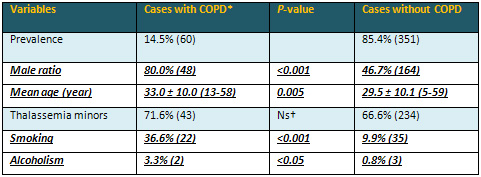
*Chronic obstructive pulmonary disease †Nonsignificant
(P>0.05)
Table 2: Associated pathologies of the study
cases
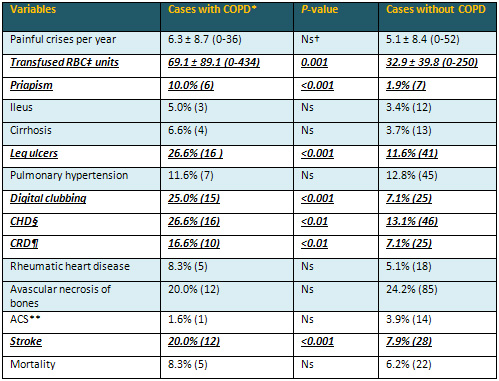
*Chronic obstructive pulmonary disease †Nonsignificant
(P>0.05) ‡Red blood cell §Coronary
heart disease Chronic renal disease **Acute chest
syndrome
Table 3: Peripheric blood values of the study
cases
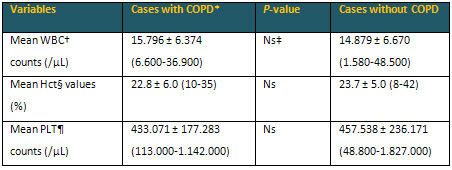
*Chronic obstructive pulmonary disease †White
blood cell ‡Nonsignificant (P>0.05) §Hematocrit
Platelet
Chronic
endothelial damage may be the most common type
of vasculitis, and the leading cause of aging
in human beings. Physical inactivity, weight gain,
smoking, alcohol, prolonged infections, and chronic
inflammatory processes such as SCDs, rheumatologic
disorders, and cancers accelerate the process.
Probably whole afferent vasculature including
capillaries are mainly involved in the process.
Much higher BP of the afferent vasculature may
be the major underlying cause, and efferent endothelium
are probably protected due to the much lower BP
in them. Secondary to the chronic endothelial
damage, inflammation, and fibrosis, vascular walls
become thickened, their lumens are narrowed, and
they lose their elastic natures that reduce the
blood flow and increase BP further. Although early
withdrawal of the causative factors may prevent
terminal consequences, after development of cirrhosis,
COPD, CRD, CHD, PAD, or stroke, the endothelial
changes may not be reversed completely due to
the fibrotic natures of them (11).
SCDs are life-threatening genetic disorders affecting
around 100,000 individuals in the United States
(12). As a difference from other causes of chronic
endothelial damage, the SCDs may keep vascular
endothelium particularly at the capillary level
(13), since the capillary system is the main distributor
of the hard RBCs to the tissues. The hard cells
induced chronic endothelial damage, inflammation,
edema, and fibrosis build up an advanced atherosclerosis
in much younger ages of the patients. As a result,
average lifespans of the patients were 48 years
in females and 42 years in males in the literature
(14), whereas they were 33.6 and 30.8 years in
the present study, respectively. The great differences
may be secondary to delayed initiation of hydroxyurea
therapy and inadequate RBC supports in severe
crises in our country. On the other hand, longer
lifespan of females with the SCDs (14) and longer
overall survival of females in the world (15)
cannot be explained by the atherosclerotic effects
of smoking and alcohol alone, instead it may be
explained by more physical power requiring role
of male sex in life that may terminate with an
exaggerated sickling and/or atherosclerosis all
over the body (16).
ACS is responsible for a considerable mortality
in the SCDs (17). According to the literature,
it occurs most often as a single episode, and
a past history is associated with an early mortality.
Similarly, all of 15 cases with ACS had only a
single episode, and two of them in the group without
COPD were fatal in spite of rigorous RBC and ventilation
support and antibiotic therapy in the present
study. The remaining 13 patients are still alive
without a recurrence at the end of the nine-year
follow up period. ACS is most common between the
ages of 2 to 4 years, and its incidence decreases
with aging (18). Parallel to the knowledge, its
incidence was only 3.6% among the patients with
an average age of 30.0 ± 10.1 years (range
5-59) in the present study. The decreased incidence
with aging may be due to a high mortality during
the first episode and an acquired immunity against
various antigens with aging. On the other hand,
ACS may also show inborn severity of the SCDs.
For example, its incidence is higher in severe
cases such as cases with sickle cell anemia (HbSS)
and a higher WBC count (17, 18). Probably, ACS
is a complex event, and the terminology of 'ACS'
does not indicate a definite diagnosis but reflects
clinical difficulty of defining a distinct etiology
in the majority of such episodes. One of the major
clinical problems lies in distinguishing between
infection and infarction, and in establishing
clinical significance of fat embolism. For example,
ACS did not show an infectious etiology in 66%
of episodes in the above studies (17, 18). Similarly,
12 of 27 episodes of ACS had evidence of fat embolism
as the cause in another study (19). But according
to our nine-year experiences, the increased metabolic
rate during infections may terminate with ACS.
In other words, ACS may be a complex sequel characterized
by disseminated endothelial damage and fat embolism
at the capillary level, not in the pulmonary vasculature
alone, instead all over the body. A preliminary
result from the Multi-Institutional Study of Hydroxyurea
in the SCDs indicating a significant reduction
of ACS episodes with hydroxyurea suggests that
a substantial number of episodes are secondary
to capillary inflammation and edema (20). Similarly,
we strongly recommend hydroxyurea therapy for
all patients and that may also be a cause of the
low incidence of ACS among our follow up cases.
Additionally, some authors showed that antibiotics
do not shorten the clinical course (21, 22), and
RBC support must be given whenever there is evidence
of clinical deterioration. RBC support has the
obvious benefits of decreasing sickle cell concentration
directly, and suppressing bone marrow for production
of the abnormal cells. So they prevent further
sickling induced damage to the lungs and other
organs. RBC support should be given early in the
course since it has prophylactic benefit. According
to our experiences, simple RBC transfusions are
superior to exchange. First of all, preparation
of one or two units of RBC suspensions each time,
rather than preparation of six units or higher
gives time to prepare more units by preventing
sudden death of such cases. Secondly, transfusions
of one or two units of RBC suspensions each time
will decrease the severity of pain, and relax
anxiety of the patients and their relatives in
a short period of time. Thirdly, transfusion of
RBC suspensions in secondary health centers may
prevent some deaths that have developed during
transport to tertiary centers for exchange.
COPD is the third leading cause of death with
various underlying causes, worldwide (23). It
is an inflammatory disease mainly affecting the
pulmonary vasculature, and smoking, excess weight,
and aging may be the major causes. As also seen
in the present study, regular alcohol consumption
may also take place in the inflammatory process.
Similarly, COPD was one of the most frequent diagnoses
in patients with alcohol dependence in another
study (24). Additionally, 30-day readmission rate
was higher in COPD patients with alcoholism (25).
Probably the accelerated atherosclerotic process
is the main structural background of functional
changes characteristic of the disease. The endothelial
process is enhanced by release of various chemicals
by inflammatory cells, and terminates with atherosclerosis,
fibrosis, and pulmonary losses. Although COPD
may mainly be an accelerated atherosclerotic process
of the pulmonary vasculature, there are several
reports about coexistence of an associated endothelial
inflammation all over the body (26, 27). For instance,
it was shown in a previous study that there may
be close relationships between COPD, CHD, PAD,
and stroke (28). Similarly, two-thirds of mortality
were caused by cardiovascular diseases and lung
cancers, and CHD was the most common one among
them in a multi-center study performed on 5,887
smokers (29). When the hospitalizations were researched,
the most common causes were the cardiovascular
diseases again (29). In another study, 27% of
all mortality were due to the cardiovascular causes
in the moderate and severe COPD patients (30).
As also observed before (31), COPD may be one
of the terminal endpoints of the SCDs due to the
higher prevalence of priapism, leg ulcers, digital
clubbing, CHD, CRD, and stroke in the SCDs cases
with COPD.
Smoking may have a major role in systemic atherosclerotic
processes such as COPD, digital clubbing, cirrhosis,
CRD, PAD, CHD, stroke, and cancers (11, 32). Its
atherosclerotic effects are the most obvious in
Buerger's disease and COPD. Buerger's disease
is an inflammatory process terminating with obliterative
changes in small and medium-sized vessels, and
it has never been reported in the absence of smoking.
Smoking induced endothelial damage probably affects
pulmonary vasculature much more than other organs
due to the higher concentration of its products
in the respiratory system. But it may even cause
cirrhosis, CRD, PAD, CHD, stroke, and cancers
with the transport of its products in the blood.
COPD may also be accepted as a localized Buerger's
disease of the lungs. Beside the strong atherosclerotic
effects, smoking in human beings and nicotine
administration in animals may be associated with
some weight loss (33). There may be an increased
energy expenditure during smoking (34), and nicotine
may decrease caloric intake in a dose-related
manner (35). Nicotine may lengthen intermeal time,
and decrease amount of meal eaten (36). Body mass
index (BMI) seems to be the highest in former,
the lowest in current, and medium in never smokers
(37). Similarly, smoking may also show the weakness
of volition to control eating, and prevalences
of HT, DM, and smoking were the highest in the
highest triglyceride having group as a significant
parameter of the metabolic syndrome (38). Additionally,
although CHD was detected with similar prevalences
in both sexes, smoking and COPD were higher in
males against the higher prevalences of BMI and
its consequences including dyslipidemia, HT, and
DM in females (32). Probably tobacco smoke induced
acute inflammation on vascular endothelium all
over the body is the major cause of loss of appetite,
since the body doesn't want to eat during fighting.
On the other hand, when we thought some antidepressant
properties of smoking and alcohol, the higher
prevalences of them in males may also indicate
some additional stresses on male sex and shortened
survival of them.
Digital changes may help to identify some systemic
disorders in the body. For instance, digital clubbing
is characterized by loss of normal <165°
angle between the nailbed and fold, increased
convexity of the nail fold, and thickening of
the whole distal finger (39). Some authors found
clubbing in 0.9% of all patients admitted to the
department of internal medicine (8), whereas the
prevalence was 4.2% in the same department in
our university (11). The exact cause and significance
is not known but chronic tissue hypoxia induced
vasodilation and secretion of growth factors have
been proposed (40-43). In the above study, only
40% of clubbing cases turned out to have significant
underlying diseases while 60% remained well over
the subsequent years (8). But according to our
experiences, digital clubbing is frequently associated
with smoking and pulmonary, cardiac, or hepatic
disorders that are featuring with chronic tissue
hypoxia. Lungs, heart, and liver are closely related
organs that affect their functions in a short
period of time. Similarly, digital clubbing may
be an indicator of disseminated atherosclerosis
particularly at the capillary level in the SCDs,
and we observed clubbing in 9.7% of patients with
the SCDs in the present study. In addition to
the SCDs, the higher prevalences of smoking (P<0.001)
and clubbing (P<0.001) in the COPD group may
also indicate some additional roles of smoking
and COPD on digital clubbing.
Leg ulcers are seen in 10 to 20% of patients with
the SCDs (44), and the ratio was 13.8% in the
present study. The incidence increases with age,
and they are also common in HbSS cases and in
males (44). Similarly, leg ulcers were found as
19.3% in males versus 8.0% in females (P<0.001)
in the present study. Beside that, mean ages of
the patients with leg ulcers were higher than
the patients without (34.8 versus 29.2 years,
P<0.000). The leg ulcers have an intractable
nature, and around 97% of healed ulcers relapse
in a period of one year (45). As a proof of their
atherosclerotic natures, the leg ulcers occur
in distal areas with less collateral blood flow
in the body (45). The hard RBCs induced chronic
endothelial damage particularly at the capillary
level may be the major cause in the SCDs (44).
Prolonged exposure to the hard cells due to blood
pooling in the lower extremities may also explain
the leg but not arm ulcers in the SCDs. As also
detected in venous ulcers of the legs, venous
insufficiency may also accelerate the process
by causing pooling of causative hard cells in
the legs. Probably pooling of blood in the lower
extremities may also have effects in the diabetic
ulcers, Buerger's disease, digital clubbing, and
onychomycosis. Beside the hard cells, smoking
and alcohol may also have some additional effects
for the leg ulcers since both of them are much
more common in males, and their atherosclerotic
effects are more obvious in COPD, Buerger's disease,
and cirrhosis (44). According to our experiences,
prolonged resolution of leg ulcers with hydroxyurea
may also suggest that they may be secondary to
increased WBC and PLT counts induced disseminated
endothelial inflammation and edema particularly
at the capillary level.
Stroke is also a common complication of the SCDs
(46). Similar to the ACS and leg ulcers, it is
more common in the HbSS cases and in cases with
a higher WBC count (47, 48). Sickling induced
disseminated endothelial damage and activations
of WBC and PLTs may terminate with chronic endothelial
inflammation, edema, and fibrosis in the brain
(49). Stroke of the SCDs may not have a macrovascular
origin instead disseminated endothelial inflammation
and edema may be much more prominent at the capillary
level. Infections, inflammations, and various
stresses may precipitate stroke since increased
metabolic rate during such events may precipitate
sickling and endothelial edema. Similar to the
ACS and leg ulcers, a significant reduction of
stroke with hydroxyurea may also suggest that
a significant proportion of stroke is secondary
to increased WBC and PLT counts induced disseminated
endothelial edema in the diseases (13, 20).
As a conclusion, SCDs cause severe chronic endothelial
damage particularly at the capillary level, and
terminate with accelerated atherosclerosis induced
end-organ failures in early years of life. Probably
ACS is a sudden onset event without any chronic
inflammatory background in the SCDs.
1. Eckel RH, Grundy SM, Zimmet
PZ. The metabolic syndrome. Lancet 2005; 365:
1415-1428.
2. Helvaci MR, Kaya H, Seyhanli M, Yalcin A. White
coat hypertension in definition of metabolic syndrome.
Int Heart J 2008; 49: 449-457.
3. Helvaci MR, Aydogan A, Akkucuk S, Oruc C, Ugur
M. Sickle cell diseases and ileus. Int J Clin
Exp Med 2014; 7: 2871-2876.
4. Helvaci MR, Acipayam C, Aydogan A, Akkucuk
S, Oruc C, Gokce C. Acute chest syndrome in severity
of sickle cell diseases. Int J Clin Exp Med 2014;
7: 5790-5795.
5. Global strategy for the diagnosis, management
and prevention of chronic obstructive pulmonary
disease 2010. Global initiative for chronic obstructive
lung disease (GOLD).
6. Castro O, Brambilla DJ, Thorington B, Reindorf
CA, Scott RB, Gillette P, et al. The acute chest
syndrome in sickle cell disease: incidence and
risk factors. The Cooperative Study of Sickle
Cell Disease. Blood 1994; 84: 643-649.
7. Fisher MR, Forfia PR, Chamera E, Housten-Harris
T, Champion HC, Girgis RE, et al. Accuracy of
Doppler echocardiography in the hemodynamic assessment
of pulmonary hypertension. Am J Respir Crit Care
Med 2009; 179: 615-621.
8. Vandemergel X, Renneboog B. Prevalence, aetiologies
and significance of clubbing in a department of
general internal medicine. Eur J Intern Med 2008;
19: 325-329.
9. Schamroth L. Personal experience. S Afr Med
J 1976; 50: 297-300.
10. Mankad VN, Williams JP, Harpen MD, Manci E,
Longenecker G, Moore RB, et al. Magnetic resonance
imaging of bone marrow in sickle cell disease:
clinical, hematologic, and pathologic correlations.
Blood 1990; 75: 274-283.
11. Helvaci MR, Aydin LY, Aydin Y. Digital clubbing
may be an indicator of systemic atherosclerosis
even at microvascular level. HealthMED 2012; 6:
3977-3981.
12. Yawn BP, Buchanan GR, Afenyi-Annan AN, Ballas
SK, Hassell KL, James AH, et al. Management of
sickle cell disease: summary of the 2014 evidence-based
report by expert panel members. JAMA 2014; 312:
1033-1048.
13. Helvaci MR, Aydin Y, Ayyildiz O. Hydroxyurea
may prolong survival of sickle cell patients by
decreasing frequency of painful crises. HealthMED
2013; 7: 2327-2332.
14. Platt OS, Brambilla DJ, Rosse WF, Milner PF,
Castro O, Steinberg MH, et al. Mortality in sickle
cell disease. Life expectancy and risk factors
for early death. N Engl J Med 1994; 330: 1639-1644.
15. Mathers CD, Sadana R, Salomon JA, Murray CJ,
Lopez AD. Healthy life expectancy in 191 countries,
1999. Lancet 2001; 357: 1685-1691.
16. Helvaci MR, Ayyildiz O, Gundogdu M. Gender
differences in severity of sickle cell diseases
in non-smokers. Pak J Med Sci 2013; 29: 1050-1054.
17. Poncz M, Kane E, Gill FM. Acute chest syndrome
in sickle cell disease: etiology and clinical
correlates. J Pediatr 1985; 107: 861-866.
18. Sprinkle RH, Cole T, Smith S, Buchanan GR.
Acute chest syndrome in children with sickle cell
disease. A retrospective analysis of 100 hospitalized
cases. Am J Pediatr Hematol Oncol 1986; 8: 105-110.
19. Vichinsky E, Williams R, Das M, Earles AN,
Lewis N, Adler A, et al. Pulmonary fat embolism:
a distinct cause of severe acute chest syndrome
in sickle cell anemia. Blood 1994; 83: 3107-3112.
20. Charache S, Terrin ML, Moore RD, Dover GJ,
Barton FB, Eckert SV, et al. Effect of hydroxyurea
on the frequency of painful crises in sickle cell
anemia. Investigators of the Multicenter Study
of Hydroxyurea in Sickle Cell Anemia. N Engl J
Med 1995; 332: 1317-1322.
21. Charache S, Scott JC, Charache P. ''Acute
chest syndrome'' in adults with sickle cell anemia.
Microbiology, treatment, and prevention. Arch
Intern Med 1979; 139: 67-69.
22. Davies SC, Luce PJ, Win AA, Riordan JF, Brozovic
M. Acute chest syndrome in sickle-cell disease.
Lancet 1984; 1: 36-38.
23. Rennard SI, Drummond MB. Early chronic obstructive
pulmonary disease: definition, assessment, and
prevention. Lancet 2015; 385: 1778-1788.
24. Schoepf D, Heun R. Alcohol dependence and
physical comorbidity: Increased prevalence but
reduced relevance of individual comorbidities
for hospital-based mortality during a 12.5-year
observation period in general hospital admissions
in urban North-West England. Eur Psychiatry 2015;
30: 459-468.
25. Singh G, Zhang W, Kuo YF, Sharma G. Association
of Psychological Disorders With 30-Day Readmission
Rates in Patients With COPD. Chest 2016; 149:
905-915.
26. Danesh J, Collins R, Appleby P, Peto R. Association
of fibrinogen, C-reactive protein, albumin, or
leukocyte count with coronary heart disease: meta-analyses
of prospective studies. JAMA 1998; 279: 1477-1482.
27. Mannino DM, Watt G, Hole D, Gillis C, Hart
C, McConnachie A, et al. The natural history of
chronic obstructive pulmonary disease. Eur Respir
J 2006; 27: 627-643.
28. Mapel DW, Hurley JS, Frost FJ, Petersen HV,
Picchi MA, Coultas DB. Health care utilization
in chronic obstructive pulmonary disease. A case-control
study in a health maintenance organization. Arch
Intern Med 2000; 160: 2653-2658.
29. Anthonisen NR, Connett JE, Enright PL, Manfreda
J; Lung Health Study Research Group. Hospitalizations
and mortality in the Lung Health Study. Am J Respir
Crit Care Med 2002; 166: 333-339.
30. McGarvey LP, John M, Anderson JA, Zvarich
M, Wise RA; TORCH Clinical Endpoint Committee.
Ascertainment of cause-specific mortality in COPD:
operations of the TORCH Clinical Endpoint Committee.
Thorax 2007; 62: 411-415.
31. Helvaci MR, Erden ES, Aydin LY. Atherosclerotic
background of chronic obstructive pulmonary disease
in sickle cell patients. HealthMED 2013; 7: 484-488.
32. Helvaci MR, Aydin Y, Gundogdu M. Smoking induced
atherosclerosis in cancers. HealthMED 2012; 6:
3744-3749.
33. Grunberg NE, Greenwood MR, Collins F, Epstein
LH, Hatsukami D, Niaura R, et al. National working
conference on smoking and body weight. Task Force
1: Mechanisms relevant to the relations between
cigarette smoking and body weight. Health Psychol
1992; 11: 4-9.
34. Walker JF, Collins LC, Rowell PP, Goldsmith
LJ, Moffatt RJ, Stamford BA. The effect of smoking
on energy expenditure and plasma catecholamine
and nicotine levels during light physical activity.
Nicotine Tob Res 1999; 1: 365-370.
35. Hughes JR, Hatsukami DK. Effects of three
doses of transdermal nicotine on post-cessation
eating, hunger and weight. J Subst Abuse 1997;
9: 151-159.
36. Miyata G, Meguid MM, Varma M, Fetissov SO,
Kim HJ. Nicotine alters the usual reciprocity
between meal size and meal number in female rat.
Physiol Behav 2001; 74: 169-176.
37. Laaksonen M, Rahkonen O, Prattala R. Smoking
status and relative weight by educational level
in Finland, 1978-1995. Prev Med 1998; 27: 431-437.
38. Helvaci MR, Kaya H, Gundogdu M. Association
of increased triglyceride levels in metabolic
syndrome with coronary artery disease. Pak J Med
Sci 2010; 26: 667-672.
39. Myers KA, Farquhar DR. The rational clinical
examination. Does this patient have clubbing?
JAMA 2001; 286: 341-347.
40. Uppal S, Diggle CP, Carr IM, Fishwick CW,
Ahmed M, Ibrahim GH, et al. Mutations in 15-hydroxyprostaglandin
dehydrogenase cause primary hypertrophic osteoarthropathy.
Nat Genet 2008; 40: 789-793.
41. Toovey OT, Eisenhauer HJ. A new hypothesis
on the mechanism of digital clubbing secondary
to pulmonary pathologies. Med Hypotheses 2010;
75: 511-513.
42. Alam MT, Sheikh SS, Aziz S, Masroor M. An
unusual side effect of interferon alfa 2A: digital
clubbing. J Ayub Med Coll Abbottabad 2008; 20:
165-166.
43. Fomin VV, Popova EN, Burnevich EZ, Kuznetsova
AV. Hippocratic fingers: clinical importance and
differential diagnosis. Klin Med (Mosk) 2007;
85: 64-68.
44. Minniti CP, Eckman J, Sebastiani P, Steinberg
MH, Ballas SK. Leg ulcers in sickle cell disease.
Am J Hematol 2010; 85: 831-833.
45. Trent JT, Kirsner RS. Leg ulcers in sickle
cell disease. Adv Skin Wound Care 2004: 17; 410-416.
46. Gueguen A, Mahevas M, Nzouakou R, Hosseini
H, Habibi A, Bachir D, et al. Sickle-cell disease
stroke throughout life: a retrospective study
in an adult referral center. Am J Hematol 2014;
89: 267-272.
47. Majumdar S, Miller M, Khan M, Gordon C, Forsythe
A, Smith MG, et al. Outcome of overt stroke in
sickle cell anaemia, a single institution's experience.
Br J Haematol 2014; 165: 707-713.
48. Helvaci MR, Aydogan F, Sevinc A, Camci C,
Dilek I. Platelet and white blood cell counts
in severity of sickle cell diseases. Pren Med
Argent 2014; 100: 49-56.
49. Kossorotoff M, Grevent D, de Montalembert
M. Cerebral vasculopathy in pediatric sickle-cell
anemia. Arch Pediatr 2014; 21: 404-414.
|
