|
Role of Power
Doppler Ultrasonography in Detection of Subclinical
Hyperuricemia in Patients with Non-Hodgkin's Lymphoma
......................................................................................................................................................................
Safaa Sayed (1)
Wafaa Gaber (2)
Waleed Hammam (3)
Ahmad Al-Ghitany (4)
Ahmed H. K. Abdelmaksoud (5)
(1) Associate Professor,
Rheumatology department, Cairo university, Egypt
(2) Assistant Professor, Rheumatology department,
Cairo university, Egypt
(3) Lecturer, Oncology department, Cairo university,
Egypt
(4) Lecturer, Internal medicine department (nephrology
unit), Ain Shams university, Egypt
(5) Lecturer,interventional Radiology department,
Cairo university, Egypt
Correspondence:
Safaa Sayed, MD
Associate Professor in Rheumatology and Rehabilitation
department,
Faculty of medicine, Cairo University
Manawat,
Giza
Egypt
Phone: 0020238171015
Email: dr.safaa_sayed@yahoo.com
|
ABSTRACT
Objective: This study aimed to detect
incidence of subclinical arthritis in patients
with NHL and the diagnostic ability of PDUS
in detecting subclinical hyperuricemia.
Methods: We studied 100 NHL patients (divided
into 2 groups depending on the presence
of the double contour (DC) sign detected
by PDUS) and 100 controls in a cross sectional
study. Demographic, clinical and serological
data were evaluated. PDUS was done to all
patients and controls. Results: There was
a statistically significant difference between
the two groups regarding the presence of
subclinical hyperuricemia in group (1)(p=0.008)
who had higher s. creatinine and gouty nephropathy
(p=0.002 and p=0.001 respectively). Conclusion:
PDUS can detect subclinical
hyperuricemia and subsequent inflammatory
arthritis in NHL patients; also it serves
as a non-invasive, bedside tool.
Key words: Hyperuricemia
; gouty nephropathy; NHL and PDUS
|
The MSU crystal deposition can
be clinically expressed as gouty arthritis, tophi
formation, urate nephropathy or urolithiasis[1].
Serum urate (SU) concentration represents the
balance between the breakdown of purines and the
rate of uric acid renal excretion. The solubility
threshold is approximately 7 mg/dl, and when exceeded
level of interstitial fluids become oversaturated,
which in turn increases the likelihood of monosodium
urate (MSU) crystal tissue deposition [2].
Increased turnover of malignant cells results
in an increase in cell lysis, catabolism of nucleic
acids, and release of purine metabolites. Renal
insufficiency develops as a consequence of hyperuricemia
and is characterized by urine supersaturated with
uric acid and crystallization of uric acid in
the renal tubules and distal collecting system
[3]. Tumor lysis syndrome (TLS), a potentially
life-threatening complication characterized by
hyperuricemia, hyperphosphatemia, hyperkalemia,
and hypocalemia can result in acute renal failure.
Patients with myeloproliferative disorders, lymphoid
malignancies, or solid tumors with large tumor
burdens are at increased risk of TLS as a consequence
of chemotherapy, corticosteroids, radiation therapy,
or stem cell transplantation [4].
Non- Hodgkin's lymphomas (NHL) are the most common
occurring hematological malignancies in the world.
They represent about 4% of all new cancer cases
and are the fifth leading cause of cancer death
[5]. The etiology of NHL is unknown although several
genetic factors, environmental and infectious
agents have been associated with the development
of lymphoma as the association with EPV, HIV and
HCV cannot be neglected[6].
The treatment of NHL includes chemotherapy with
different regimens according to the type of NHL,
involved field radiotherapy and the recent target
theory as Anti-CD 20 (rituximab) and Anti-CD 52
(alemtuzumab) antibodies [7].
Ultrasound (US) has been demonstrated to be a
valid imaging modality to detect musculoskeletal
involvement in patients with gout [8,9]. The main
US findings related to MSU crystal deposition
include hyperechoic enhancement of the superficial
margin of the hyaline cartilage ; double contour
(DC) sign, hyperechoic spots within tendons and
soft tissues, tophi and bone erosions [10]. Additionally,
an increase of blood flow surrounding the MSU
deposits detected by power Doppler (PD) has been
described as an indicator of inflammatory activity
[11,12].
One hundred Egyptian patients
diagnosed as NHL were consecutively recruited
from oncology department of Cairo university hospitals
(46% males and 54% females, ) were included in
the present study. They were classified into 2
groups, according to the presence of (DC) sign
detected by power Doppler ultrasonography (PDUS);
group (1) had DC sign (47/100) (47%), and group
(2) had no DC sign (53/100) (53%). Four NHL patients
(8.5%) in group (1) gave a past history of acute
gouty arthritis that was diagnosed according to
the criteria for the classification of acute gouty
arthritis [13].
All patients were asked to complete a questionnaire
on demographics and medications used. All subjects
were informed about the aim of the study and gave
their consent. Patients were musculoskeletally
examined in the rheumatology and rehabilitation
department, Cairo university hospitals. Blood
was drawn at the time of the study for analyses
which included the following: complete blood picture,
serum creatinine, fasting blood sugar, serum uric
acid, K, P and Ca and liver functions were tested
for all enrolled cases. Plain X-ray was done for
all enrolled patients.
Power Doppler ultrasonography examination
All subjects subsequently underwent a structural
musculoskeletal US evaluation of both knees and
1st MTP joints by two experienced observers. Bilateral
knee joints (transverse suprapattellar view of
the femoral cartilage in maximal flexion) and
bilateral 1st MTP joints (longitudinal dorsal
and medial views) were examined to evaluate the
double contour sign and effusion, but no tendon
US was performed. Double contour sign was defined
as a hyper echoic band over the femoral articular
cartilage or metatarsal head cartilage using a
12.5 MHz linear probe (Philips-ATL®, HDI 5000,
Philips®, Bothell, WA, USA). Blood flow was
examined with a pulse repetition frequency of
750 KHz and a Doppler frequency between 6 and
8 MHz. Attention was given not to compress the
tissues under examination to avoid a "blanching"
of the PD signal due to the transducer pressure.
Statistical analysis
Computer software package SPSS 15 was used in
the analysis for quantitative variables, mean
(as a measure of central tendency) and standard
deviation (as measures of variability). Frequency
and percentages were presented for qualitative
variables.
ANOVA test was used to estimate differences in
quantitative variables. Chi-square and Fisher-exact
tests were used to estimate differences in qualitative
variables. P Value < 0.05 is significant [14].
Kappa statistics were calculated to determine
the proportion of inter- and intra-observer agreement
beyond that expected by chance. The method for
estimating an overall kappa value in cases of
multiple observers and categories is based on
the work of Landis and Koch (1) A value of k
= 1.0 corresponds to complete agreement;
0, no agreement; and less than 0, disagreement.
Landis and Koch suggested that a kappa value <
0.20 indicates slight agreement; 0.21-0.40, fair
agreement; 0.41-0.60, moderate agreement; 0.61-0.80,
substantial agreement; and 0.81-1.00, almost perfect
agreement [15].
Figure 1: the right side shows
longitudinal view of the 1st metatarsophalangeal
joint, and left side shows transverse view of
knee joint, both show the double contour sign
(arrow)
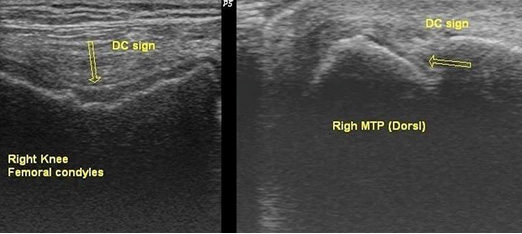
Figure 2: the right side shows longitudinal
view of the 1st metatarsophalangeal joint with
mild effusion and punched out erosion (arrow),
and left side shows longitudinal view of knee
joint with effusion and synovial hyper-vascularity
(arrow)
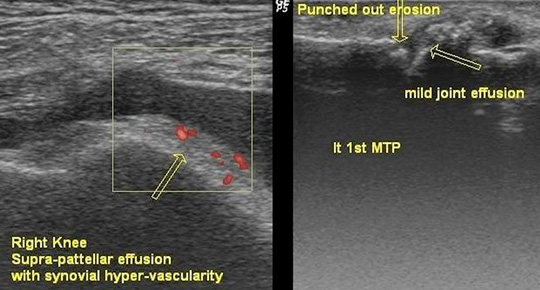
Figure 3: shows that patients in group (1)
had significantly higher SUA than those of group
(2) (P0.008)
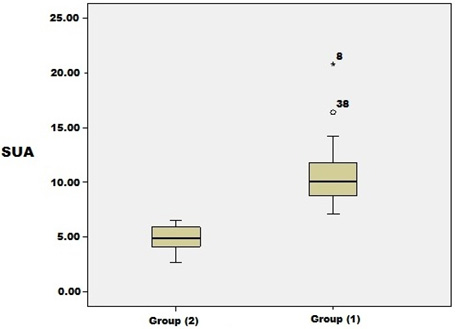
Figure 4: shows that patients in group (1)
significantly had higher serum creatinine (P0.002)
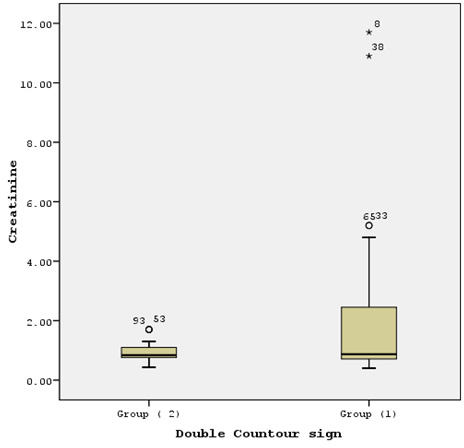
Table 1: Demographics and medications used by
the studied patients
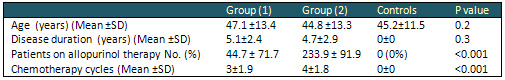
* Statistically significant value (p< 0.05)
Table 2: Clinical, laboratory and PDUS data
of the current patients and controls
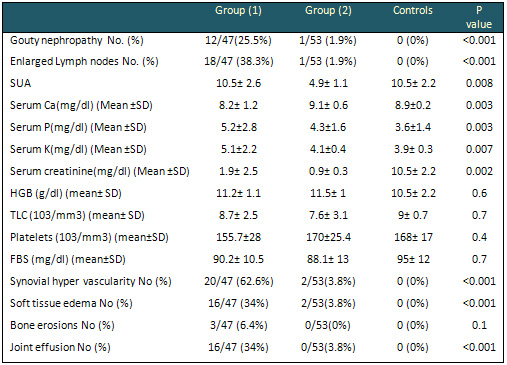
SUA: serum uric acid, Ca: calcium , P : phosphorus
, K : potassium, HGB: hemoglobin, TLC: total leukocyte
count, FBS : fasting blood sugar, FBS: fasting
blood sugar .
* Statistically significant value (p< 0.05)
One hundred NHL patients (46% were males and 54%
were females) and100 age matched healthy controls
with a mean age of 45.2±11.5years were
examined during this study. They were classified
into two groups according to the presence of the
DC sign as shown in Figure 1; inter and intra-reader
analysis is 0.71 and 0.74 respectively. Demographics
and medications received
are shown in Table 1. All patients were on chemotherapy.
Clinical examination revealed MTP joint swelling
only in four patients in group 1 (4/47 (8.5%))
and absent in group 2. Joint pain was found in
seven patients; five of them complained of MTP
joints pain and 2 of them complained of knee joint
pain (6/47 (12.8%) in group 1 and 1/53 (18.9%)
in group 2.
Tumor lysis syndrome was present in 10/47 (21.3%)
NHL patients in group 1 and absent in group 2)
(P< 0.001). PDUS detected synovial hyper vascularity
in (62.6%) in group 1 and joint effusion in (34%)
in group 1 as shown in Figure 2; other clinical,
laboratory and PDUS parameters are shown in Table
2.
On comparing the two examined groups, it was found
that patients in group 1 had higher SUA (p=0.008)
as shown in Figure 3, and higher serum creatinine
(P0.002) as shown in Figure 4. Gouty nephropathy
was present in group 1 in 12/47 (25.5%); two of
them (16.7%) were on hemodialysis but only 1/53
(1.9%) in group 2 had gouty nephropathy with highly
significant difference (P<0.001).
In group 1 only 16/47 (34%) were taking allopurinol,
in comparison to 49/53 (92.5%) in group 2 with
highly significant difference (p<0.001) (odds
ratio =0.04 and 95% CI ranging between 0.01-0.13).
There was a past history of acute
gouty arthritis in 4/47 (8.5%) in group 1 and
absent in group 2 with significant difference
(P0.04). Plain -X ray radiography of the patients
with past history of acute gouty arthritis revealed
soft tissue edema.
Gout is one of the commonest
forms of inflammatory arthritis. The prevalence
appears to be rapidly increasing worldwide [16].
It is mediated by the crystallization of uric
acid within the joints [17]. Urate crystals are
deposited predominantly in the superficial portions
of the articular cartilage. These characteristic
cartilaginous deposits are not readily demonstrated
with conventional diagnostic imaging modalities[18]
. Articular chondrocalcinosis is also a common
crystal deposition joint disease in which calcium
pyrophosphate dihydrate (CPPD) crystals deposit
within the joint cartilage and fibrocartilage.
It appears by US as punctate hyper echoic dots
within the cartilage resembling "rosary beads"[19].
Treatment of NHL results in metabolic disturbances
that require urgent treatment, among these, hyperuricemia
has emerged as an important complication associated
with the use of newer therapeutic agents. Allopurinol,
a xanthine oxidase inhibitor, has traditionally
been used to treat hyperuricemia; it blocks the
production of uric acid from xanthine and hypoxanthine
without affecting the breakdown of already formed
uric acid, and at the same time prevents new production
[20]. This coincides with the results in the present
study as the number of patients on allopurinol
therapy is much higher in group 2 who presented
with lesser complications of (SUA).
In the current study, on comparing the two groups
it was found that patients with DC sign had significant
hyperuricemia than those without DC sign (P0.008).
Double contour sign represents SUA crystals deposition
in the hyaline cartilages [21]. As confirmation
of the presence of MSU in the hyaline cartilage,
Thiele and Schlesinger [22] demonstrated the disappearance
of the double contour sign in patients with gout
successfully treated with urate-lowering agents
who had maintained SU levels below 6 mg/dl for
at least 7 months [23 ]. This may strengthen the
need for treatment necessity in asymptomatic individuals
with hyperuricemia and indisputable US features
of MSU crystal tissue deposition such as the double
contour sign or the presence of tophi [24].
PDUS detected synovial hyper vascularity in (62.6%)
in group 1 and (3.8%) in group 2 and soft tissue
edema in (34%) in group 1 and (34%) in group 2
with highly significant difference (P<0.001);
joint effusion was detected in (34%) in group
1 and was absent in group 2 with highly significant
difference (P<0.001). This strengthens the
importance of use of PDUS in detecting the subclinical
attacks arthritis [23].
On comparing the two examined groups, it was found
that patients in group 1 had higher serum creatinine
(P0.002), as gouty nephropathy was present in
group 1 in12/47; (25.5%) two of them (16.7%) are
on hemodialysis, but only 1/53 (1.9%) in group
2 with highly significant difference (P<0.001).
In group 1 only 16/47 (34%) were taking allopurinol,
in comparison to 49/53 (92.5%) in group 2 with
highly significant difference (P<0.001) (odds
ratio =0.04 and 95% CI ranging between 0.01-0.13).
It was found that allopurinol blocks the formation
of uric acid by inhibiting the enzyme xanthine
oxidase, thus causing an increase in plasma concentrations
of the uric acid precursors hypoxanthine and xanthine.
Patients at high risk for tumor lysis still need
to excrete the preexisting uric acid that is not
targeted by allopurinol. Allopurinol also inhibits
de novo purine synthesis, further lowering uric
acid concentrations [25].
PDUS can detect the subclinical
hyperuricemia and the attacks of subclinical arthritis.
Also the use of allopurinol therapy decreases
the SUA level in NHL patients and subsequently
the incidence of gouty arthritis and gouty nephropathy.
1. Yamamoto T. Definition and
classification of hyperuricemia. Nippon Rinsho.
2008;66:636-40.
2. Edwards NL. The role of hyperuricemia and gout
in kidney and cardiovascular disease. Cleve Clin
J Med. 2008;75(Suppl 5):S13-S16.
3. Davidson WB, Thakkar S, Hix JK, et al. Pathophysiology,
clinical consequences, and treatment of tumor
lysis syndrome. Am J Med 2004;116:546-54.
4. Bruce D. Cheson, MD, and Bonni S. Dutcher,
PhD : Managing Malignancy-Associated Hyperuricemia
with Rasburicase. Georgetown University, Lombardi
Comprehensive Cancer Center, Washington, DC. J
Support Oncol 2005;3:117-24.
5. JemalA, Siegel R, Ward E. et al., Cancer statistics
2007 CA. Cancer J clin 2007;57:43.
6. Chiu BC and Weisenburger DD. An update of the
epidemiology of the NHL clin lymphoma. 2003 ;
4: 161.
7. Romaguera JE, Fayad L., Rodriguez MA., et al:
High rate of durable remission after treatment
of mantle cell lymphoma with Mabthera- hyper C
VAD. J clin oncol. 2005;23:7013.
8. Filippucci E, Scirè CA, Delle Sedie
A. et al. Ultrasound imaging for the rheumatologist.
XXV. Sonographic assessment of the knee in patients
with gout and calcium pyrophosphate deposition
disease. Clin Exp Rheumatol. 2010;28:2-5.
9. Dalbeth N, McQueen FM. Use of imaging to evaluate
gout and other crystal deposition disorders. Curr
Opin Rheumatol. 2009;21:124-31.
10. Thiele RG, Schlesinger N. Diagnosis of gout
by ultrasound. Rheumatology (Oxford) 2007;46:1116-21.
11. Puig JG, de Miguel E, Castillo MC.et al: Impact
of ultrasonography. Nucleosides Nucleotides Nucleic
Acids. 2008;27:592-95
12. Wright SA, Filippucci E, McVeigh C.et al.
High-resolution ultrasonography of the first metatarsal
phalangeal joint in gout: a controlled study.
Ann Rheum Dis. 2007;66:859-64.
13. Wallace SL, Robinson H, Masi AT, et al. Preliminary
criteria for the classification of the acute arthritis
of primary gout. Arthritis Rheum 1977;20:895-900,
with permission of the American College of Rheumatology.
14. Dawson B, Trapp RG. Basic and clinical biostatistics.
3rd ed. McGraw-Hill Inc; 2001.
15. Landis JR, Koch GG. The measurement of observer
agreement for categorical data. Biometrics. 1977
33:159-174.
16. Zaka R, Williams CJ. New developments in the
epidemiology and genetics of gout. Curr Rheumatol
Rep.2006;8:215-23.
17. Choi HK, Curhan G. Gout: epidemiology and
lifestyle choices. Curr Opin Rheumatol 2005;17:341-45.
18. Sokoloff L.. The pathology of gout. Metabolism
1957;6:230-43.
19. Filippucci E, Scire CA, Delle Sidie A, Iagnocco
A, Riente L, Meenagh G. Ultrasound imaging for
the rheumatologist , XXV: sonographic assessment
of the knee in patients with gout and calcium
pyrophosphate deposition disease . Clin Exp Rheumatolo
2010;28:2-5
20. Betül Sevinir, Metin Demirkaya, Birol
Baytan, Adalet Meral Gunes. : Hyperuricemia and
tumor lysis syndrome in children with non-Hodgkin's
lymphoma and acute lymphoblastic leukemia. Uluda?
University, Bursa, Turkey. Turk J Hematol 2011;
28: 52-9
21. Burt HM, Dutt YC. Growth of monosodium urate
monohydrate crystals: effect of cartilage and
synovial fluid components on in vitro growth rates.
Ann Rheum Dis. 1986;45:858-64.
22. Thiele RG, Schlesinger N. Ultrasonography
shows disappearance of monosodium urate crystal
deposition on hyaline cartilage after sustained
normouricemia is achieved. Rheumatol Int. 2010;30:495-503.
23. Carlos Pineda , Luis M Amezcua-Guerra, Carla
Solano, Pedro Rodriguez-Henríquez, Cristina
Hernández-Díaz, Angelica Vargas,
et al: Joint and tendon subclinical involvement
suggestive of gouty arthritis in asymptomatic
hyperuricemia: an ultrasound controlled study.
PMCID: PMC3241349.Published online 2011 January
24. Neogi T. Asymptomatic hyperuricemia: perhaps
not so benign? J Rheumatol. 2008;35:734-37.
25. Holdsworth MT, Nguyen P. Role of i.v. allopurinol
and rasburicase in tumor lysis syndrome. Am J
Health Syst Pharm 2003;60:2213-24.
|
