|
A study to compare
the safety and efficacy of levofloxacin versus
cefuroxime axetil in patients with uncomplicated
lower UTI in a North Indian Medical College and
Hospital.
......................................................................................................................................................................
Preeti Garg (1)
Amandeep Singh (2)
Rani Walia (3)
Harbir Kaur Rao (4)
Prithpal Singh Matreja (2)
Amarjit Kaur Gill (5)
PML Khanna (6)
(1) Assistant Professor, MBBS, MD, Department
of Pharmacology, Gian Sagar Medical College and
Hospital, Village Ram Nagar, District Patiala,
Punjab, India-140601
(2) Associate Professor, MBBS, MD, Department
of Pharmacology, Gian Sagar Medical College and
Hospital, Village Ram Nagar, District Patiala,
Punjab, India-140601
(3) Professor and Head, MBBS, MD, Department of
Pharmacology, Maharishi Markendeshwar Institute
of Medical Sciences and Research, Mullana, Ambala,
India-133207
(4) Professor, MBBS, MD, Department of Medicine,
Gian Sagar Medical College and Hospital, Village
Ram Nagar, District Patiala, Punjab, India-140601
(5) Professor and Head, MBBS, MD, Department of
Microbiology, Adesh institute of Medical Sciences
and Research, Bathinda, Punjab, India-151109
(6) Professor & Head, MBBS, DA, MD, Department
of Pharmacology, Gian Sagar Medical College and
Hospital, Village Ram Nagar, District Patiala,
Punjab, India-140601
Correspondence:
Dr. Preeti Garg, Assistant Professor, Department
of Pharmacology, Gian Sagar Medical College and
Hospital, Village Ram Nagar, District Patiala,
Punjab, India-140601
Mobile: +91-8146265728
Email: preetigarg@gmail.com
|
ABSTRACT
Background and Objectives: Uncomplicated
lower UTI accounts for around 150 million
cases worldwide, every year. Antibiotics
commonly used for the treatment of uncomplicated
lower UTI include fluoroquinolones, trimethoprim-sulphamethoxazole,
nitrofurantoin, cephalosporins, and amoxicillin.
No comparative study between levofloxacin
and cefuroxime axetil in patients with uncomplicated
lower UTI could be searched. So, this randomized
study was designed to compare the efficacy
and tolerability of levofloxacin 500 mg
once daily with cefuroxime axetil 250 mg
twice daily in the treatment of uncomplicated
lower UTI in adult Indian patients.
Methods: This prospective, parallel
group comparative study was conducted in
100 patients with uncomplicated lower UTI.
Patients were assessed for clinical and
bacteriological success over the study period.
Results: 89 patients of the total
of 100 patients enrolled in the study completed
the study. E.coli was the most common organism
isolated in both the groups. Patients in
levofloxacin group showed improvement in
clinical symptoms by 95.35 percent, as compared
to 89.13 percent in the cefuroxime group.
However, the intergroup difference was not
statistically significant (p>0.05). Levofloxacin
group showed decrease in bacteriological
scoring by 95.35 percent, and cefuroxime
group showed decrease by 86.96 percent.
The difference in bacteriological scoring
between the two treatment groups was not
significant (p>0.05).
Conclusion: The results of our study
show that cefuroxime axetil in a dose of
250 mg twice daily and levofloxacin 500
mg once daily for three days, are equally
efficacious in treating patients with uncomplicated
lower UTI. The comparative clinical and
bacteriological successes between the two
groups were statistically not significant,
and both drugs were well-tolerated by the
patients.
|
Urinary tract infections (UTI)
include a heterogeneous group of clinical syndromes
and diseases with a worldwide incidence of at
least 150 million cases annually. (1) UTI can
be broadly divided into lower UTI, which involves
urethra, bladder; and upper UTI that involves
kidney, ureter, and prostate. Patients with lower
UTI present with features of frequency of micturition,
dysuria, urgency, suprapubic pain and tenderness,
foul smelling urine and hematuria(2), whereas
patients of upper UTI present with loin pain and
tenderness, fever and systemic upset.(3)
Escherichia coli are the most common organism
(71 to 78 percent) causing uncomplicated UTI,
followed by Proteus (4-12 percent), Klebsiella,
Enterococcus faecalis and occasionally Pseudomonas
and Staphylococcus.(2,4,5) Diagnosis of UTI depends
on the symptoms and urine culture. Treatment of
acute, uncomplicated lower UTI includes mainly
oral or parenteral antibiotics. Antibiotics commonly
used for the treatment of uncomplicated lower
UTI include fluoroquinolones, trimethoprim-sulphamethoxazole,
nitrofurantoin, aminoglycosides, cephalosporins,
and amoxicillin.(5,6,7,8)
Levofloxacin, the S-isomer of ofloxacin is active
against a wide range of gram negative and gram
positive organisms including Staphylococcus spp.,
Streptococcus, H. influenzae, Escherichia coli,
Klebsiella spp, Proteus, Pseudomonas aeruginosa
and atypical bacteria accountable for causing
lower UTI.(9, 10) Comparative studies in lower
UTI have demonstrated similar or significantly
better results with levofloxacin versus ciprofloxacin,
norfloxacin or ofloxacin, and other conventionally
used antibiotics e.g. amoxicillin, trimethoprim-sulphamethoxazole
(TMP-SMX).(5,11,12,13) The drug levofloxacin is
well-absorbed, its bioavailability approaches
100 percent, and it is widely distributed throughout
the body.(14) The drug is well-tolerated with
a low incidence of resistance.(15,16)
Cefuroxime axetil, an oral second generation broad
spectrum cephalosporin is also effective against
Gram positive and Gram negative bacteria including
Staphylococcus spp., Streptococcus, Niesseria,
E.coli, Klebsiella, Proteus responsible for causing
lower UTI, but not Pseudomonas aeruginosa. Cefuroxime
also is well-tolerated, with incidence of resistance
similar to levofloxacin and much lower as compared
to TMP-SMX and amoxicillin.(15, 17,18) Studies
in patients with acute uncomplicated lower UTI
treated with cefuroxime axetil, show overall cure
rate ranging from 86 percent to 97 percent (19,20)
In another study, at one week post therapy, 88
percent of the patients in the cefuroxime axetil
group were clinically and bacteriologically cured.(21)
Naber and Koch reported a multicentre study done
on 163 women with acute uncomplicated lower UTI,
with clinical cure and improvement seen in 84.8
percent and 95.2 percent of patients treated with
125 mg cefuroxime axetil twice daily for three
days and 100 mg ofloxacin twice daily for three
days, respectively.(18) Seven to nine days after
therapy, bacteriuria had been eliminated in 80.3
percent and 89.1 percent of the patients receiving
cefuroxime axetil and ofloxacin respectively.
No comparative study between levofloxacin and
cefuroxime axetil in patients with uncomplicated
lower UTI could be searched. So, this randomized
study was designed to compare the efficacy and
tolerability of levofloxacin with cefuroxime axetil
in the treatment of uncomplicated lower UTI in
adult Indian patients.
Study design and population
This prospective, randomized, comparative, open-label,
parallel group study was done in 100 patients
suffering with uncomplicated lower UTI visiting
the outpatient medicine department of Government
Medical College and Hospital, Patiala during the
period from 2006 to 2007; conducted in association
with department of medicine, microbiology and
pharmacology.
Patients of either sex, between 18 to 60 years
of age, suspected to have uncomplicated UTI due
to typical symptoms of dysuria, frequency, and/or
urgency, sensitivity to both levofloxacin and
cefuroxime axetil and willing to give written
informed consent were included in the study. Patients
with signs and symptoms of complicated UTI (fever,
flank pain, costovertebral tenderness), pregnancy,
diabetes, epilepsy, abnormalities of urinary tract,
UTI within the last two weeks, use of antibiotics
within the last 3 days, history of hypersensitivity
reaction to the test drugs, or unable to give
informed consent were excluded from the study.
The study was approved by the Institutional Ethics
Committee.
Patients visits to the medicine OPD were planned
as per the following schedule: During the first
baseline visit (Visit 1), detailed history and
clinical examination of the patient were performed
and urine sample was sent for microscopic examination,
culture and sensitivity. The next visit was planned
after 2 days (Visit 2), when the urine culture
and sensitivity report became available. Based
on urine culture and sensitivity report, patients
were randomized into group A and group B. Patients
in Group A were prescribed tablet levofloxacin
500 mg once daily for 3 days whereas Group B received
tablet cefuroxime axetil 250 mg twice daily for
3 days. Patients were then called at the fourth
day after starting the treatment (Visit 3), when
the symptoms were recorded to assess clinical
improvement and urine sample was sent for microscopic
examination, culture and sensitivity.
Outcome measurements
The outcome measures used for efficacy variable
were clinical success, which comprised of a sum
total of clinical cure (improvement in all three
symptoms) and clinical improvement (improvement
in one or two symptoms); and bacteriological success
(complete eradication of infecting organisms on
culture).
Statistical analysis
The results were analyzed using Fisher's exact
test and unpaired students t test, using Instat
Graphpad 3.10 version software. A p-value <0.05
was considered statistically significant.
Of the total of 100 patients
(49 on levofloxacin, i.e. Group A and 51 on cefuroxime
axetil, i.e. Group B) who were enrolled in the
study, 89 patients (43 in Group A and 46 in Group
B) completed the study. Eleven patients, six in
group A and five in group B did not come for follow-up.
The data was calculated for these 89 patients
(33 Male, 56 Female) who completed the study.
Demographic and Baseline data
At the baseline visit (Visit 1), there was no
significant difference (p>0.05) in demographic
and clinical characteristics between the two treatment
groups (Table 1). 62.92 percent (67.44 percent
in group A and 58.70 percent in group B) of the
patients were female. Increase in frequency (all
patients in both groups A and B) was the most
common symptom, whereas dysuria was the least
common symptom at baseline visit. E. Coli (74.41percent
in group A, 82.6 percent in group B) was the most
common organism in both the groups, as shown in
Table 2.
Table 1: Demographic and clinical characteristics
of the two treatment groups at baseline visit
(Visit 1)
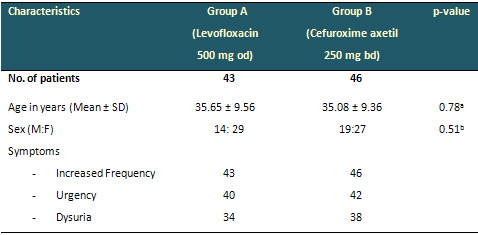
a Value determined using two-tailed unpaired student
"t" test.
b Value determined using Fisher's exact test.
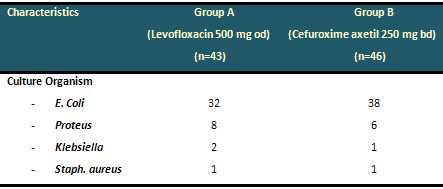
Table 2:. Distribution of organisms in the
two treatment groups seen on bacteriological culture
at visit 2
Clinical Success
At visit 3, patients in group A showed mean percentage
decrease in symptoms of increased frequency, urgency
and dysuria by 72.09 percent, 70 percent and 94.12
percent, respectively. Similarly, patients in
group B showed mean percentage decrease in symptoms
of increased frequency, urgency and dysuria by
78.26 percent, 71.43 percent and 92.11 percent,
respectively (Figure 1). Also, of the total of
43 patients in levofloxacin group A, 23 showed
clinical cure, 18 had clinical improvement and
2 patients showed no improvement in any of the
symptoms of lower UTI, thus, showing a mean percentage
improvement in clinical symptoms by 95.35 percent.
In cefuroxime group B, 22 out of the total 46
patients showed clinical cure and 19 showed clinical
improvement, however, 5 patients showed no improvement
in any of the symptoms. Thus, patients in group
B showed a mean percentage improvement in clinical
symptoms at visit 3 by 89.13 percent (Figure 2).
However, the difference between the two treatment
groups was not statistically significant (p>0.05),
although levofloxacin (95.35 percent versus 89.13
percent) decreased clinical success scores slightly
more than cefuroxime (Figure 2).

Figure 1: Changes in clinical symptoms among
the treatment groups after 3-days treatment
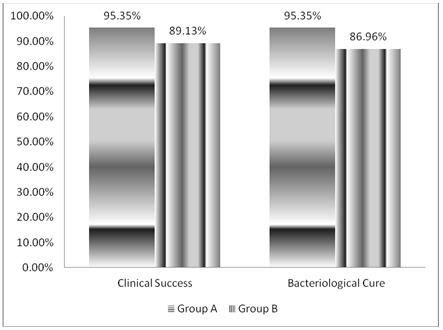
Figure 2: Comparison in clinical success and
bacteriological success seen among the treatment
groups
Bacteriological Success
Mean percentage improvement in bacteriological
success score, from baseline to visit 3 of the
study period was 95.35 percent for group A (41
had bacteriological cure, 2 had bacteriological
failure), and 86.96 percent for group B (40 had
bacteriological cure, 6 had bacteriological failure),
as shown in Figure 2. The inter-group difference
between the two treatment groups A and B was not-significant
(p>0.05), although levofloxacin decreased bacteriological
scores slightly more than cefuroxime.
Safety
Of the 89 patients who completed the study, only
three patients (6.98 percent) in the levofloxacin
group developed adverse effects with the drug.
Two patients (4.65 percent) in levofloxacin group
reported nausea and one patient (2.33 percent)
complained of headache with the drug. Of the patients
on cefuroxime, two patients (4.35 percent) complained
of nausea with the drug. The comparison in the
incidence of adverse effects between the two treatment
groups was statistically non-significant (p=0.67),
and was done using Fisher's exact test.
Urinary tract infections (UTI)
are among the most common bacterial infections
and the treatment of UTI is aimed at improvement
of clinical symptoms and eradication of infection.
In uncomplicated acute lower UTI, short-course
three-day therapy with cefuroxime axetil or levofloxacin
antibiotics is found to be effective, as shown
by various studies.(13)
The results of our study show that E.coli was
the most common pathogen isolated, similar to
the findings seen in other studies.(2,4,5) Also,
cefuroxime axetil in a dose of 250 mg twice daily
and levofloxacin 500 mg once daily were found
to be equally efficacious in treating patients
with uncomplicated lower UTI. There was no statistically
significant difference (p > 0.05) between the
clinical and bacteriological success rates of
the two treatment groups, and both drugs were
well-tolerated by the patients. The levofloxacin
group showed slightly better response than cefuroxime
axetil, maybe because fluoroquinolones are known
to have superior action than cephalosporins against
gram negative organisms responsible for causing
uncomplicated lower UTI.
In a study by Richard et al, the clinical success
rate for levofloxacin vs ofloxacin was 98.1 percent
versus 97 percent and bacteriological success
rate was 96 percent with levofloxacin and 93 percent
for ofloxacin. Our study showed similar response
to levofloxacin, although the dose of levofloxacin
used in this study was 500 mg od, as compared
to 250 mg od in the previous study.(13) In a study
by Lee et al in 2011, the susceptibility of E.coli
to levofloxacin was 77.5 percent.(22)
The current study shows the effect of cefuroxime
axetil was also quite similar to that seen in
previous studies. In a study by Naber et al, the
clinical success rate for cefuroxime axetil vs
ofloxacin was 84.8 percent vs 95.2 percent and
bacteriological success rate was 80.3 percent
with cefuroxime axetil and 89.1 percent for ofloxacin.(18)
The dose of cefuroxime axetil used in this study
was 125 mg twice daily for 3 days. Our study was
quite similar and showed clinical success rate
89.13 percent and bacteriological success rate
86.96 percent to be slightly better, probably
as the dose used was 250 mg twice daily. Another
study where patients were prescribed cefuroxime
axetil 125 mg twice daily for 7 days, showed clinical
success and bacteriological success rate to be
97 percent.(23) The study by Lee et al shows 86.1
percent susceptibility of E. coli to cefuroxime.(22)
In another study the susceptibility of E. coli
to oral cefuroxime was 68.6 percent versus 97.1
percent to parenteral cefuroxime.(24)
Our study revealed that the two drugs were well
tolerated when used for three day therapy. The
adverse events of nausea and headache with the
test drugs resolved in a few hours in both treatment
groups.(12,18) No patient withdrew from the study
because of adverse effects, showing good tolerance
to study drugs. The adverse effects were lesser
in our study in both the groups as compared to
earlier studies.
In conclusion, our study shows both drugs cefuroxime
axetil 250 mg twice daily and levofloxacin 500
mg once daily to be effective in the three-day
treatment of patients with uncomplicated lower
UTI, with no statistically significant difference
between the efficacy of cefuroxime axetil and
levofloxacin, although levofloxacin showed slightly
better response than cefuroxime axetil. Both the
drugs were well-tolerated.
There are certain limitations in our study: First,
more number of patients in each group would make
the results more significant. Second, prolonged
follow-up visit would have revealed better any
cases of relapse or treatment failure.
1. Norrby R. Urinary tract
infections. In: Ausiello D, Goldman L, eds. Cecil
Textbook of Medicine. Philadelphia. Saunders Elsevier;
2004:1909-13.
2. Yaqoob MM. Renal Disease. In: Kumar P, Clark
M, ed. Clinical Medicine. Edinburg. Saunders Elsevier;
2009: 571-647.
3. Tolkoff-Rubin NE, Cotran RS, Rubin RH. Urinary
tract infection, pyelonephritis, and reflux nephropathy.
In: Brenner BM, ed. Brenner & Rector's The Kidney.
8th ed. Vol. 2. Philadelphia, Saunders Elsevier;
2008: 1203-38.
4. Kosakai N, Kumamoto Y, Hirose T, Tanaka N, Hikichi
Y, Shigeta S et al. Comparative studies on activities
of antimicrobial agents against causative organisms
isolated from urinary tract infections (1987). I.
Susceptibility distribution. Jpn J Antibiot 1990;43(6):919-53.
5. Thabet L, Messadi AA, Medde B, Mbarek M, Turki
A, Redjeb SB. Bacteriological profile of urinary
tract infections in women in Aziza Othmana Hospital:
495 cases. Tunis Med 2010;88(12):898-901.
6. Bush LM, Chaparro-Rojas F, Okeh V, Etienne J.
Cumulative clinical experience from over a decade
of use of levofloxacin in urinary tract infections:
critical appraisal and role in therapy. Infect Drug
Resist 2011;4:177-189.
7. Schaeffer AJ. The expanding role of fluoroquinolones.
Am J Med 2002;113(Suppl 1A):45S-54S.
8. Tierney LM. Urologic Disorders. In: McPhee SJ,
Papadakis MA, eds. Current Medical Diagnosis and
Treatment. New York: McGraw-Hill; 2008:816-36.
9. Petri WA, Jr. Sulfonamides, trimethoprim-sulfamethoxazole,
quinolones, and agents for urinary tract infections.
In: Brunton LL, Lazo JS, Parker KL, eds. Goodman
& Gilman's The Pharmacological Basis of Therapeutics.
New York. Mc Graw-Hill; 2006:1111-26.
10. Chambers HF, Deck DH. Sulfonamides, trimethoprim
& quinolones. In: Katzung BG, Masters SB, Trevor
AJ, eds. Basic and clinical pharmacology. New Delhi.
Tata McGraw-Hill; 2009:815-22.
11. Tanaka K, Iwamoto M, Maesaki S, Koga H, Kohno
S, Hara K, et al. Laboratory and clinical studies
on levofloxacin. Jpn J Antibiot 1992;45(5):548-56.
12. Barry AL, Fuchs PC, Brown SD. In vitro activities
of five fluoroquinolone compounds against strains
of Streptococcus pneumoniae with resistance to other
antimicrobial agents. Antimicrob Agents Chemother
1996;40(10):2431-33.
13. Richard GA, De Abate CA, Ruoff GE, Corrado M,
Fowler CL, Morgan N. A double blind, randomized
trial of the efficacy and safety of short course,
once-daily levofloxacin versus ofloxacin in uncomplicated
urinary tract infections. Infect Dis Clin Pract
1998;9:323-29.
14. Scheen AJ. Pharma-clinics. The drug of the month.
Levofloxacin (Tavanic). Rev Med Liege 2000;55(11):1015-17.
15. Aypak C, Altunsoy A, Düzgün N. Empiric
antibiotic therapy in acute uncomplicated urinary
tract infections and fluoroquinolone resistance:
a prospective observational study. Ann Clin Microbiol
Antimicrob 2009;8:27.
16. Von Rosentiel N, Adam D. Quinolone antibacterials.
An update of their pharmacology and therapeutic
use. Drugs 1994;48(2):326.
17. Akram M, Shahid M, Khan AU. Etiology and antibiotic
resistance patterns of community-acquired urinary
tract infections in J N M C Hospital Aligarh, India.
Annals of Clinical Microbiology and Antimicrobials
2007;6:4.
18. Naber KG, Koch EM. Cefuroxime axetil versus
ofloxacin for short-term therapy of acute uncomplicated
lower urinary tract infections in women. Infection
1993;21(1):34-9.
19. Williams KJ, Hebblethwaite EM, Brown GW, Cox
DM, Plested SJ. Cefuroxime axetil in the treatment
of uncomplicated UTI: a comparison with cefaclor
and augmetin. Drugs Exp Clin Res 1987;13(2):95-9.
20. Leigh DA, Joy GE, Tait S, Harris K, Walsh B.
Treatment of acute uncomplicated urinary tract infections
with single daily doses of cefuroxime axetil. J
Antimicrob Chemother 1989;23(2):267-73.
21. Iravani A, Richard GA. Single-dose cefuroxime
axetil versus multiple-dose cefaclor in the treatment
of acute urinary tract infections. Antimicrob Agents
Chemother 1989;33(8):1212-16.
22. Lee SJ, Lee DS, Choe HS, Shim BS, Kim CS, Kim
ME, et al. Antimicrobial resistance in community-acquired
urinary tract infections: results from the Korean
Antimicrobial Resistance Monitoring System. J Infect
Chemother 2011;17(3):440-6.
23. Cooper J, Raeburn A, Brumfitt W, Hamilton-Miller
JM. Comparative efficacy and tolerability of cephradine
and cefuroxime axetil in the treatment of acute
dysuria and/or frequency in general practice. Br
J Clin Pract. 1992;46(1):24-7.
24. Farrell DJ, Morrissey I, De Rubeis D, Robbins
M, Felmingham D. A UK multi-centre study of the
antimicrobial susceptibility of bacterial pathogens
causing urinary tract infection. J Infect 2003;46(2):94-100.
|
