|
Nitric Oxide and
Oxidative Stress Properties of L-Carnitine in
Diabetic Hypertensive Rats Biochemical & Histological
Study
......................................................................................................................................................................
Mona Abd El-Latif
Abuzahra (1)
Sherifa Abd-Elsalam Mustafa (2)
K.S.A. Taif University
Faculty of Medical Applied Science
Department of Medical Biotechnology and Medical
Laboratory
(1) Ain Shams University
Hospital, Faculty of Medicine, Department of Clinical
Pathology
(2) Benha University, Faculty of Medicine, Department
of Histology and Cytology
Correspondence:
Mona Abd El-Latif Abuzahra
Ain Shams University Hospital, Faculty of Medicine,
Department of Clinical Pathology
Phone No.+966542496717
Email: monabuzahra@hotmail.com
|
ABSTRACT
Background: Diabetes mellitus and
hypertension are a global health problem
due to their serious complications, which
along with oxidative stress have been shown
to contribute to endothelial dysfunction.
Aim: The aim of the present study
was to investigate the role of the beneficial
effect of L-carnitine in healthy and streptozotocin
(STZ) N -nitro-L-arginine
methyl ester (L-NAME) induced diabetes mellitus
and hypertension in rats. -nitro-L-arginine
methyl ester (L-NAME) induced diabetes mellitus
and hypertension in rats.
Results: Results showed that diabetic
hypertensive (DH) rats had significant increase
in the level of plasma glucose, malondialdhyde
(MAD), cholesterol (CH), triglycerides (TG),
urea, creatinine, and the activity of serum
liver enzymes (AST, ALT) compared to normal
control rats. Blood glutathione (GSH) content
and erythrocyte superoxide dismutase (SOD)
activity and nitric oxide level (NO) were
significantly lowered. Supplementation of
L- carnitine for 6 weeks improved plasma
glucose, lipids, liver and kidney functions.
In addition, both normal healthy rats and
DH rats treated with L- carnitine showed
increase in blood GSH and SOD activity and
serum nitrate level (stable product of nitric
oxide NO) as compared with healthy controls
and DH respectively. Histopathological and
immunochemical study of heart confirmed
the biochemical results.
Conclusion: It was concluded that
administration of L-carnitine reduces or
delays oxidative stress in diabetic hypertensive
rats.
Key words: L-carnitine - L-NAME-
Nitric Oxide, Oxidative Stress, Diabetic
rats, Hypertensive rats, Histological Study.
|
Diabetes results in a state
of increased reactive oxygen species (ROS) production,
and oxidative stress is implicated in the development
and progression of various diabetic complications
(Baynes, 1991;Yao et al. 2009). Increased oxidative
stress is thought to play an important role in
the etiology and pathogenesis of chronic complications
of diabetes (Scott and King, 2004; Yao et al.,
2009).
The antidiabetic actions of individual free amino
acids are of great interest. Intervention of glycation
may prove to be beneficial to patients suffering
from diabetes mellitus. Free amino acids are known
to mitigate the glycation of lens protein, delay
cataractogenesis and bring down blood sugar levels
in diabetic rats and promote tissue sensitivity
towards insulin. Further, amino acids inhibit
the binding of glucose with proteins, the first
step in the pathway of glycation cascade by competitive
inhibition, thereby offering protection (Anuradha,
2009).
Arterial hypertension is associated with a high
production of reactive oxygen species and a decrease
in the antioxidant defence systems. Since oxidative
stress has gained importance in the last few years
as one of the mechanisms involved in the origin
and development of hypertension, and considering
that L-carnitine (LC) is a useful compound in
different pathologies characterized by increased
oxidative status, the aim of this work was to
test the hypothesis that LC might protect the
heart against hypertension-induced oxidative damage.
In spite of a wide range of drugs being available
in the market, treatment of arterial hypertension
still remains a challenge, and new therapeutic
strategies could be developed in order to improve
the rate of success in controlling this disease
(Zambrano et al., 2013).
The importance of L-carnitine (b-hydroxy-y-N-trimethyammonium
butyric acid) for the lipid and carbohydrate metabolism
has been long established. Carnitine is required
to transport long -chain fatty acids from the
cytoplasm to the mitochondrial matrix where their
oxidation occurs, and on the other hand, carnitine
increases the sensitivity of the cells to insulin
and the use of glucose by the peripheral tissues
(Mamoulakis et al., 2004).
Some other effects of carnitine on cellular metabolism
as protection against oxygen free radicals and
of mitochondrial biogenesis in aged rats were
demonstrated. Carnitine influence membrane fluidity,
ion channel function, and smooth muscle contractility.
It was suggested that membrane effects are implicated
in the mechanism by which carnitine derivatives
protect the heart from ischemia or oxidative stress.
This might be in concert with findings on the
changes of cardiac carnitine metabolism in various
hypertensive models (Rauchova, 1998).
Nitric oxide (NO) is the most pivotal molecule
secreted by endothelium and thus is a major mediator
of endothelial function. The production of NO
is catalyzed by a family of enzymes called nitric
oxide synthases (NOS), which convert the amino
acid L-arginine to L-citrulline and NO, apart
from playing an important role in vasodilation.
Evidence suggests that NO plays a major role in
regulating blood pressure and glucose levels,
and thus impaired NO bioactivity forms an important
component of hypertension and diabetes. The physiological
importance of NO in the regulation of blood pressure
is evidenced by the fact that pharmacological
inhibition of NO synthases leads to severe hypertension,
vascular injury, and glomerulosclerosis in experimental
animals (Shiekh et al., 2011).
Experimental design and animal
grouping
Design: 40 white male albino rats weighing 150-200
g. were used for this study. All animals were
housed in stainless steel cages, 10 per cage under
controlled environmental conditions. Diabetes
was induced in male Wister albino rats by single
intraperitoneal injection of 50 mg/kg streptozotocin
(STZ) (Heo et al.,2002). Four days after STZ injection,
rats received N -nitro-L-arginine
methylester (L-NAME) (0.5 mg/mL in drinking water
for four weeks) for induction of hypertension
(Zambrano et. al., 2013). L-carnitine (0.5 g/100
gm diet) was given daily to DH rats for six weeks,
respectively (Oka et al., 2008). -nitro-L-arginine
methylester (L-NAME) (0.5 mg/mL in drinking water
for four weeks) for induction of hypertension
(Zambrano et. al., 2013). L-carnitine (0.5 g/100
gm diet) was given daily to DH rats for six weeks,
respectively (Oka et al., 2008).
Our work was carried out in accordance with the
guidelines of Faculty of Applied Medical Science
at Taif University in K.S.A. for animal use.
Handling of the animal was the same for all groups
and did not affect weight gain.
Groups:
1- Group 1: Normal control: 10 rats fed the
balanced diet during the entire study (10 weeks).
2- Group 2: L-carnitine: 10 rats fed the
balanced diet supplemented with L-carnitine during
the entire study (10 weeks).
3- Group 3: Diabetic hypertensive group
(DH): this group contains 10 diabetic hypertensive
rats.
4- Group 4: Diabetic hypertensive group
treated with L-carnitine (DH+L-car): 10 diabetic
hypertensive rats receive L-carnitine as treatment
for 6 weeks.
Our goal is to achieve a diabetic hypertensive
model in 4 weeks following treatment period for
6 weeks. This model provided us with a reliable
method and resembles the clinical cases of diabetic
hypertensive rats and their treatment. After six
weeks treatment, heart rate and blood pressure
of all animals were recorded by the tail cuff
method using the Harvard Blood Pressure Monitor.
Blood samples were collected from the tail vein
of lightly anesthetized overnight fasted animals
from all the groups.
Sample collection and analytical methods
By the end of the experimental periods, blood
samples were collected in two centrifuge tubes.
One tube contained heparinized blood used for
determination of SOD and GSH activity. In the
second tube serum was separated by leaving blood
sample for 15 minutes at a temperature of 25 oC
then it was centrifuged for 20 minutes at 4000
r.p.m., using clean dry disposable plastic syringes
and stored at -20°C for subsequent biochemical
measurements as follows: lipid profile (TC, TG)
(Fossati and Prencipe, 1982), liver enzyme activities
related to its function (ALT, AST) (Reitman and
Frankel, 1957), kidney function (urea and creatinine)
(Li, 1996), heart biomarkers (LDH, CK) (Würzburg
et al., 1977), glucose (Trinder,1969) Lipid peroxidation
and oxidative stress were measured through malondialdehyde
(MDA), nitric oxide (NO), oxidative stress markers,
reduced glutathione (GSH) and superoxide dismutase
activity (SOD) according to Mahesh et al. (2009).
Tissues were analyzed using commercial kits routine
(Bancroft and Gamble, 2002), histological methods
H&E stain.
Histological Examination:
Heart specimens from each group were examined
histopathologically. Heart tissues were fixed
in 10% formalin solution for 48 hours and embedded
in paraffin wax, sectioned (4 um),
and then stained with hematoxylin and eosin. Pathological
changes were evaluated in the tissues as previously
described (Helin HO 2006), De Rossi A 2007).
Immunohistochemical staining:
Immunohistochemistry was conducted on paraffin
embedded heart sacrifices from control and treated
rats. Slides were deparaffinized, dehydrated,
washed in phosphate buffer saline then covered
with peroxide block staining (PBS) to block off
endogenous staining and incubated for 10 minutes
at room temperature in a humidity chamber. Monoclonal
mouse caspase-3 antibody (Lab vision, USA) (Cai,
L 2002) was applied on the tissue sections then
incubated horizontally in a humidity chamber for
an hour, at room temperature. After removal of
excess buffer, the sections were incubated in
preformed strept avidin peroxidase. DAB substrate-
chromogen (3.3- Diaminobenzidine tetrahydrochloride)
was applied on slides for 5-15 minutes until the
desired brown color was obtained. Counter - staining
was done by using Mayer's haematoxylin.
Image Analysis
The data were obtained using Lecia Qwin 500 image
analyzer computer system (England). The image
analyzer consisted of a colored video camera,
colored monitor, hard disc of IBM personal computer
connected to the microscope, and controlled by
Lecia Qwin 500 software. The image analyzer was
first calibrated automatically to convert the
measurement units (pixels) produced by the image
analyzer program into actual micrometer units.
The area and area % of caspase-3 immunoexpression
in myocardial sections of the animals of all groups
were measured using an objective lens of magnification
10, i.e. of total magnification of 100. Ten random
fields were measured for each specimen. In each
chosen field, the areas of caspase-3 immunoactivity
were enclosed inside a standard measuring frame
(Figure 1).
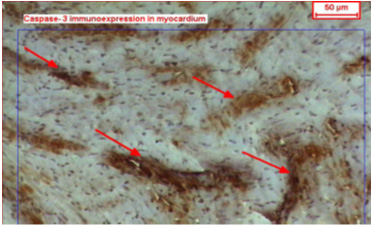
Figure 1: A copy of display seen on the monitor's
screen of the image analyzer, showing +ve caspase-3
immunoreactivity in the myocardium
Statistical Analysis
Statistical analysis was carried out using Graph
Pad Instat software (version 3, ISS-Rome, Italy).
Unless otherwise specified, groups of data were
compared with an unpaired t-test one-way analysis
of variance (ANOVA). Values of P < 0.05 were
regarded as significant. Data were expressed in
tables as mean ± standard error (SE) (Altman
and Bland 1996).
Effect of L- carnitine on
hypertension, heart rates and heart weight:
The current results tabulated in Table 1 administration
of L-carnitine by healthy and DH rats showed that
there is a significant decrease in hypertension
in the diabetic hypertensive L- carnitine treated
group (STZ+L-NAME +L-car) compared to the untreated
group(STZ+L-NAME) , heart rates also showed that
there is a significant amelioration after treatment
with L-carnitine (P < 0.05).
Table 1: Effect of L-carnitine (L-car) on the
blood pressure , heart rates and mean heart weight
in control and diabetic hypertensive (STZ+L-NAME)
rats
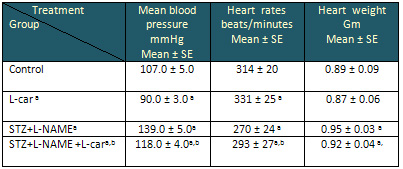
Data are presented as mean ± SE, n= 10
a and b indicate significant change from control,
STZ+L-NAME, respectively at p< 0.05
using ANOVA test.
Diabetic hypertensive rats (STZ+L-NAME), showed
decreased heart weights that was statistically
significant as compared with controls (group I).
On the other hand, in animals of (STZ+L-NAME +L-car),
the loss of heart weights was statistically insignificant
as compared with controls (Table 1).
Effect of L- carnitine on blood glucose and
lipid profile:
Results tabulated in Table 2 revealed that administration
of L-carnitine at tested doses by healthy rats
showed a non significant decrease in serum glucose
level. While administration of L-carnitine at
the same doses by DH rats showed a significant
decrease in glucose level p< 0.05. Regarding
serum CH,TG and LDL-C levels our results showed
a significant decrease in the DH+L-carnitine group
than the diabetic hypertensive group, while HDL-C
showed a significant increase P < 0.05.
Table 2: Effect of L-carnitine (L-car) on the
levels of blood glucose, triglycerides, total
cholesterol, high-density lipoprotein-cholesterol
(HDL-C) and low-density lipoprotein-cholesterol
(LDL-C) in control normal and diabetic hypertensive
(STZ+L-NAME) rat serum
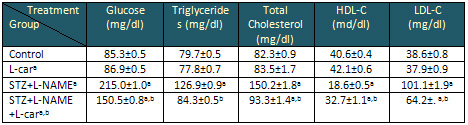
Data are presented as mean ± SE, n= 10
a and b indicate significant change from control,
STZ+L-NAME, respectively at p < 0.05
using ANOVA test.
Effect of L-carnitine on ALT, AST, LDH, CK,
urea and creatinine, in healthy and the corresponding
diabetic hypertensive rats:
The results tabulated in Table 3 illustrate the
effect of L-carnitine on serum ALT, AST, LDH,
CK, urea and creatinine, in healthy and diabetic
hypertensive rats. The results revealed that L-carnitine
supplementation to the healthy rats resulted in
a non significant change compared to untreated
healthy rats. While administration of L-carnitine
by DH rats showed a significant decrease (P <
0.05) in the levels of ALT, AST, LDH, CK, urea
and creatinine as compared with the diabetic hypertensive
control group.
Table 3: Effect of L-carnitine (L-car) on the
levels of Liver enzyme activities (ALT, AST),
cardiac enzyme activities (LDH, CK) and kidney
functions (urea, creatinine), in control normal
and diabetic hypertensive (STZ+L-NAME) rat serum
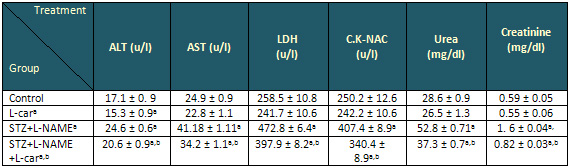
Data are presented as mean ± SE, n= 10
a and b indicate significant change from control,
STZ+L-NAME, respectively at p< 0.05
using ANOVA test.
Effect of L-Carnitine on GSH, SOD, NO and MDA
in healthy and their corresponding diabetic hypertensive
rats:
Regarding tblood (GSH) and erythrocyte (SOD) activity,
serum NO results tabulated in Table 4 revealed
that consuming L-carnitine either by healthy and
diabetic hypertensive groups showed a significant
increase in these values as compared with their
corresponding groups. Our results showed also
administration of L-carnitine by the healthy rats
(group 2) (L-car) caused a non significant reduction
in the serum level of MDA as compared with control
(group one). On the other hand, administration
of L-carnitine by DH rats resulted in a significant
reduction (P < 0.05) on serum MDA compared
with diabetic hypertensive untreated rats.
Table 4: Effect of L-Carnitine on Glutathion
reductase (GSH), superoxide dismutase (SOD), nitric
oxide (NO), and malondialdehyde (MDA) in control
normal and diabetic hypertensive (STZ+L-NAME)
rat (mean ±SE).
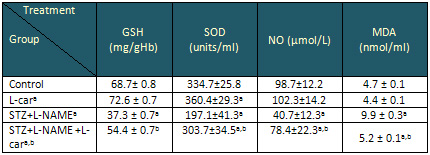
Data are presented as mean ± SE, n= 10
a and b indicate significant change from control,
STZ+L-NAME, respectively at p< 0.05
using ANOVA test.
Effect of L-Carnitine on mean area % of caspase-3
immunoreactivity in the myocardium in control
normal and diabetic hypertensive rats: Table 5
shows mean area % of caspase-3 immunoreactivity
in the myocardium of control and treated groups:
Table 5: Effect of L-Carnitine on mean area
% of caspase-3 immunoreactivity in the myocardium
in control normal and diabetic hypertensive rats.
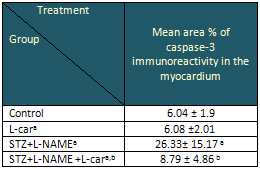
Data are presented as mean ± SE, n= 10
a and b indicate significant change from control,
STZ+L-NAME, respectively at p< 0.05
using ANOVA test.
Diabetic hypertensive treated
animals of (group III), showed increased heart
weights that was statistically highly significant
as compared with controls (group I). On the other
hand, in animals of (group IV), the loss of heart
weights was statistically insignificant as compared
with controls (Table 1). Mean area % of caspase-3
immunoreactivity in the myocardium of control
and treated groups shown in Table 5.
Light microscopic results of control heart sections
(group I) stained with H&E, the myocardium
showed branching and anastomosing muscle fibers
with centrally located oval basophilic euchromatic
nuclei and connected together by intercalated
discs (Figure 2). In L-carnitine treated control
animals (group II), the myocardium appeared nearly
with normal structure as control rats (fig.3).
Infiltrating inflammatory cells were observed
in the left ventricle sections of D H rats, and
these cells were usually found in clusters of
cells located throughout the interstitium with
congested blood vessels (Figure 4). Treatment
with L-carnitine in (group IV) showed markedly
reduced inflammatory cell infiltration in diabetic
hypertensive rats however, some inflammatory cells
were still found within the interstitial component
of the left ventricle (Figure 5).
Figure 2: A photomicrograph of a longitudinal
section of the myocardium of a control adult rat
(group I) showing branching muscle fibers with
centrally located oval nuclei (arrows). Note:
intercalated discs (lines) and flat dark nuclei
of the fibroblasts (white arrow) of the connective
tissue endomysium. (Hx. & E. X 400)
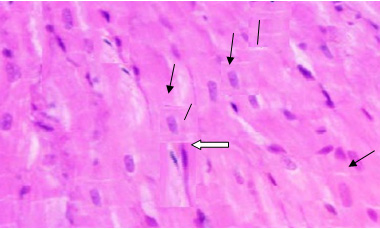
Figure 3: A photomicrograph of a longitudinal
section in the myocardium of an adult rat from
group II (L carnitine control rats), showing normal
myocardium which appeared nearly the same as control
rats. (Hx. & E. X 400)
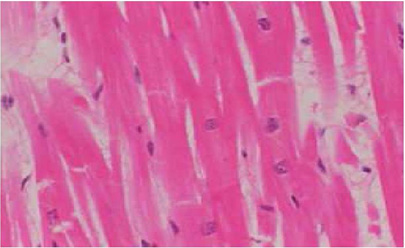
Figure 4: A photomicrograph of a longitudinal
section in the myocardium of an adult rat from
group III (diabetic hypertensive rats), showing
inflammatory cell infiltration (arrow) & congestion
of blood vessels (2 heads arrow) with some degeneration
in myocardium rats (white arrow). (Hx. & E.
X 400)
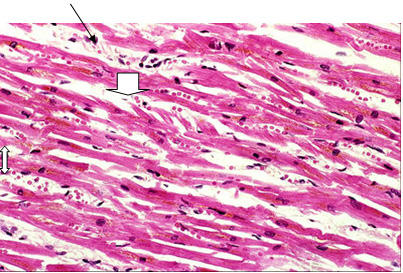
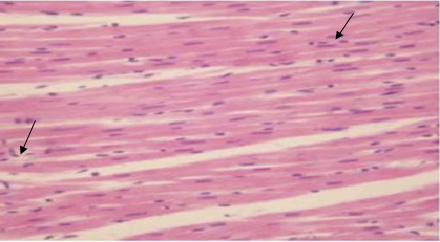
Figure 5: A photomicrograph of a longitudinal
section in the myocardium of an adult rat from
group IV (l-carnitine treated diabetic hypertensive
rats), showing myocardium with some inflammatory
cells (arrows).(Hx. & E. X 400)
Immunohistochemical and image analysis results:
Myocardial sections of control animals (group
I) showed a negative immune reaction to caspase
- 3 (Figure 6) i.e. no brownish color in the cytoplasm
(apoptosis). Group II (LC treated animals) like
controls showed a negative immune reaction to
caspase - 3 (Figure 7).
Figure 6: A photomicrograph of a longitudinal
section of the myocardium of a control adult rat
(group I) showing negative immune reaction to
caspase - 3. (Caspase-3 Immunoreactivity, X 400)
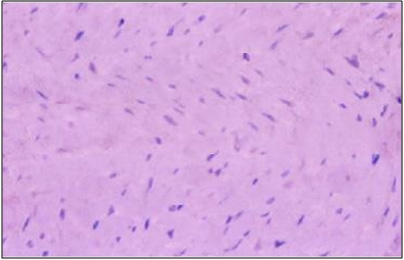
Figure 7: A photomicrograph of a longitudinal
section of the myocardium of an adult rat from
group II, (l carnitine treated animals) showing
negative immune reaction to caspase-3 (arrows).
(Caspase-3 Immunoreactivity, X 400).
Diabetic hypertensive treated animals (group III),
showed increased area of positive immune reaction
to caspase- 3 (Figure 8). In group IV, there were
few areas with positive immune reaction to caspase
- 3 (Figure 9). Diabetic hypertensive treated
animals (group III) showed a highly significant
increase in the mean area % of caspase-3 immunoreactivity
in the myocardium as compared with controls. However
the (Group VI) showed a statistically significant
decrease in the mean area % of caspase-3 expressions
(Table 5).
Figure 8: A photomicrograph of a longitudinal
section of the myocardium of an adult rat from
group III, (diabetic hypertensive animals) showing
very strong positive immune reaction to caspase-3
(arrows). (Caspase-3 Immunoreactivity, X 400)
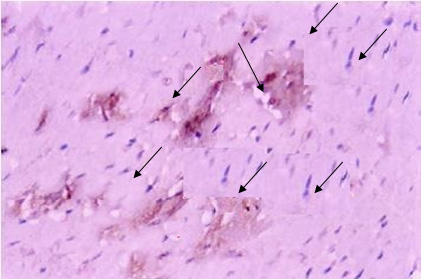
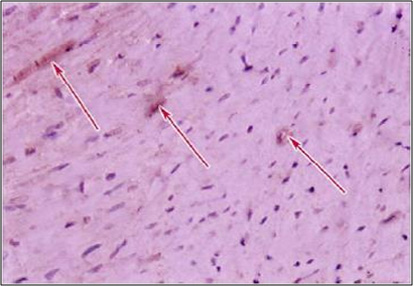
Figure 9: A photomicrograph of a longitudinal
section of the myocardium of an adult rat from
group IV, (diabetic hypertensive treated animals)
showing weak positive immune reaction to caspase-3
(arrows). (Caspase-3 Immunoreactivity, X 400)
Reduced NO availability in diabetes
mellitus and hypertension, underlines its relevance
to the development of secondary complications
in these clinical conditions. Alteration of NO
metabolism and increased oxidant stress, was demonstrated
to be involved in the pathogenesis of macrovascular
events, which are increased in hypertensives as
well as diabetics. Results of the present study
revealed that administration of L-carnitine by
healthy and diabetic hypertensive rats caused
a significant decrease in hypertension, while,
resting heart rates were increased as compared
to their corresponding controls. These results
are in agreement with Ruggenenti et al (2009)
and Malton et al (2006). They suggest, that a
deficiency of free carnitine can manifest as elevated
free fatty acids, suggesting a reduction of inter-cellular
transport. Elevating free carnitine levels in
diabetic animals with a fixed and relatively inadequate
availability of glucose as a myocardial fuel apparently
corrected the defects in myocardial function by
providing more intracellular fatty acids as an
energy substrate.
In the present study diabetic untreated rats showed
a significant elevation of serum glucose level.
Our results were confirmed by (Malton et al.,
2006) who illustrated that the blood glucose level
of diabetic rats increased compared to control
animals. Administration of L-carnitine at the
tested doses by diabetic rats only showed a significant
decrease in serum glucose level compared to the
untreated group. These results are in agreement
with Mamoulakis et al (2004) who reported that
carnitine increases the sensitivity of the cells
to insulin and the use of glucose by the peripheral
tissues. Carnitine administration improved whole
body insulin sensitivity, glucose tolerance and
prevents oxidative stress.
L-carnitine supplementation produced significant
decreases in serum TG, and T-cholesterol, LDL-C
while there was a significant increase in HDL-C
in diabetic hypertensive rats. These results are
in agreement with those of González-Ortiz
et al (2008) and El-Metwally et al (2003), who
reported that oral L-carnitine increases plasma
free carnitine levels, improves dyslipidemia and
decreases oxidative stress, with reduction of
cardiac parameters. L-carnitine administration
to diabetic hypertensive rats reduces significantly
hypertriglyceridemia (Table 2) via decreased synthesis
of triglycerides by the liver or by inhibition
of triglyceride release from the liver.
L-carnitine suppressed hydroxyl radical production
in the Fenton system, probably by chelating their
iron, is required for the generation of hydroxyl
radicals. Thus, the reduction in lipid peroxidation
in the present study might be due to the iron-chelating
property of L-carnitine. This hypothesis is consistent
with the previous study which has demonstrated
that L-carnitine showed a strong antioxidant activity
against irradiation-induced lipid peroxidation
and has free radical scavenging effects (Mansour,
2013).
In the present work diabetic hypertensive animals
showed a significant increase in the AST, ALT,
CK and LDH activities (P ?0.05), also serum urea
and creatinine levels. This may be due to increase
in oxidative damage and decrease in antioxidant
capacity of the liver which suggests that oxidative
stress has an impaction of liver disorders. These
results are in harmony with that of Khalil (2009).
The oral administration of L-carnitine (Table
3) shows that serum concentration of urea and
creatinine were significantly decreased. The effect
of L-carnitine on renal lipid metabolism could
serve as a new therapeutic approach, as it counters
the renal changes associated with metabolic syndrome.
Hence, L-carnitine has beneficial effects on renal
function.
Anuradha (2009) reported that L-carnitine protects
against liver, kidney and heart disease. L-carnitine
improves heart function in diabetics and hypertensives
and increases the level of glucose oxidation,
a process that helps cells make use of glucose.
McMackin (2007) and Ruggenenti, et.al. (2009)
reported that, Acetyl-l-carnitine safely ameliorated
arterial hypertension, insulin resistance, impaired
glucose tolerance, and hypoadiponectinemia in
subjects at increased cardiovascular risk. Whether
these effects may translate into long-term cardioprotection
is worth investigating.
In the present work, the increased serum MDA value
in diabetic hypertensive rats may be attributed
to the increased level of oxygen free radicals
which could be due to their increased production
and/or decreased destruction by non-enzymatic
and enzymatic antioxidants. Our results are in
agreement with Sailaja-Devi and Das (2005), who
reported a significant increase in the plasma
level of MDA, as well as a significant decrease
in serum level of NO(x). GSH and the activity
of SOD in RBCs lysate in diabetic animals compared
to healthy controls. Also Barakat (2006) illustrated
that the decrease in GSH levels during diabetes
is probably due to its increased utilization by
the hepatic cells. This may be due to an attempt
by the hepatocytes to counteract the increased
formation of lipid peroxides. Tas et.al. (2007)
illustrate both plasma and tissue MDA levels were
significantly reduced in the diabetic group treated
with individual free amino acids compared to those
of the diabetic untreated group. These alternations
might be related to hypolipidemic, hypoglycemic
and direct oxidative effects of free amino acids.
The antioxidative and hypoglycemic effect of L-carnitine
might also be involved in the changes in antioxidative
enzyme activities. Our results showed that erythrocyte
SOD activity was significantly increased in the
diabetic hypertensive control group and the diabetic
hypertensive group treated with L-carnitine compared
to control group; the elevation might be due to
the protective mechanism against oxidative stress.
L-carnitine effectively protects and improves
mitochondrial function in vivo: it acts as an
antioxidant, so by inhibiting ROS and RNS it protects
the vascular endothelial tissues against oxidative
damage in hypertension Gómez-Amores et
al (2007). Thus, L-carnitine treatment effectively
protected the liver tissue against oxidative damage
and showed marked improvement in its antioxidant
status.
Nitric oxide level showed a significantly reduced
level (P < 0.5) in diabetic hypertensive
rats (Table 4) than control ones. Various studies
have reported a significant decrease of plasma
nitric oxides in diabetes mellitus and hypertension;
our results coincide with these reports. Shiekh
et.al. (2011), presumed that the cascade of NO
bioactivity and availability on smooth muscle
cells was impaired in the early affected stage
of diabetes mellitus and followed the decrease
of endothelial NO production.
In the present study, the decreased serum NO value
in diabetic hypertensive rats was ameliorated
by administration of L-carnitine. Shiekh et.al.(2011),
reported that, Nitric oxide (NO) turnover is vital
for proper endothelial function to maintain a
healthy vascular system. Various risk factors
responsible for hypertension and diabetes may
disrupt this homeostasis, leading to decreased
bioavailability and/or bioactivity of NO, which
potentiates endothelial dysfunction. Plasma NO
is a useful indicator of NO homeostasis and vascular
endothelial function which plays a key role in
the development and progression of diseases like
diabetes and hypertension.
Cardiovascular remodeling includes hypertension,
endothelial damage, cardiac hypertrophy, inflammation,
ventricular contractile dysfunction and fibrosis
(Weber KT et al 2001). L-Carnitine plays a major
role, as a cofactor, in the transportation of
free fatty acids from the cytosol to the mitochondria
for adenosine triphosphate synthesis. An altered
metabolic substrate used in the failing heart
also contributes to the dysfunction of the mitochondrial
electron transport chain, resulting in enhanced
production of superoxide (Rosca MGetal 2008).
Mitochondrial dysfunction and increased mitochondrial
superoxide production, preceding endothelial dysfunction,
might favour the development of hypertension (Puddu
P et. al. 2008).
Free radicals also potentiate mitochondrial dysfunction
by further damaging mitochondrial DNA, with resultant
impairment in the synthesis of some components
of the respiratory chain and further increases
in superoxide production (Puddu P et. al. 2008,
Esposito LA et. al. 1999 Shibutani S 1991, Zorov
DB 2006). The current experiment was designed
to estimate the effect of L-carnitine on nitric
oxide and oxidative stress in normal and in diabetic
hypertensive rats. In this study Infiltrating
inflammatory cells were observed in the left ventricle
sections of D H rats, and these cells were usually
found in clusters of cells located throughout
the interstitium with congested blood vessels.
Treatment with L-carnitine in (group IV) showed
markedly reduced inflammatory cell infiltration
in diabetic hypertensive rats, however, some inflammatory
cells were still found within the interstitial
component of the left ventricle. The results of
the current investigation were strongly supported
by (Ferrari R, 2003, Malone JI,2003) who found
that L-Carnitine treatment improved heart function
after ischaemia and reperfusion injury, and also
improved heart rate regulation and ventricular
size in streptozotocindiabetic rats. In addition,
the anti-hypertensive effects of L-carnitine in
this study may result from inhibition of inflammation,
as inflammation is an integral part of the cardiovascular
remodeling observed in L-NAME hypertensive rats.
Furthermore, plasma concentrations and cardiac
expression of inflammatory markers such as IL-6
and TNFa were reduced after L-carnitine treatment
in L-NAME
Several other studies have also proved the efficiency
of L-carnitine in reducing blood pressure in patients
with pulmonary hypertension (El-Beshlawy A 2008),
in rats with L-NAME-induced hypertension (Miguel
2008) and in fructose-fed hypertensive rats (Rajasekar
P 2007). The mechanisms by which L-carnitine can
decrease blood pressure include its role in enhancing
fatty acid oxidation (El-Beshlawy A 2008) and
the consequent role to reduce the production of
superoxide (G_lÅin I 2006), and further
increasing the availability of nitric oxide (Rajasekar
P 2007). L-carnitine controls oxidative stress
by improving mitochondrial function (G_lÅin
I 2006), (Calvani M, 2000). Diabetic hypertensive
treated animals (group III) showed a highly significant
increase in the mean area % of caspase-3 immunoreactivity
in the myocardium as compared with controls. However
the (Group VI) showed decrease in the mean area
% of caspase-3 expressions in the present study.
These results were in agreement with (Abdel Baky
N, 2011), who found that l-carnitine significantly
reduced the level of heart-type fatty acid binding
protein, capase-3 activity, as well as myocardial
DNA damage in diabetic hypertensive rats.
1. Abdel Baky N, Al Rasheed
N -, Al-Rasheed N. L-Carnitine and irbesartan ameliorate
development of myocardial oxidative damage and apoptosis
in diabetic hypertensive rats. Poster abstract in
the Lancet/JACC first Asia Pacific cardiovascular
summit 2011: July 9-10, Hong Kong.
2. Altman DG and Bland JM. Statistics notes: Comparing
several groups using analysis of variance. BMJ 1996;
312: 1472-1473.
3. Anuradha CV. Amino acid support in the prevention
of diabetes and diabetic complications. Current
protein and peptide science 2009: 10: 8-17.
4. Bancroft JD and Gamble M. Theory and practice
of histological techniques 2002; 5th ed. Churchill
Livingstone.
5. Barakat LA. Antimicrobial effect and biochemical
study of some herbs on diabetic rats. Ph.D. Degree
of science, Biochemistry and Nutrition Department,
Women's College, Ain Shams Univeristy 2006.
6. Baynes J. Role of oxidative stress in development
of complications in diabetes. Diabetes 1991; 40:
405-412.
7. Cai L, Wei L, Guangw W, Luping G, Youchun J,
and Kang YJ. Hyperglycemia - induced apoptosis in
mouse myocardium. Diabetes. 2002; 51: 1938-48.
8. Calvani M, Reda E, Arrigoni-Martelli E. Regulation
by carnitine of myocardial fatty acid and carbohydrate
metabolism under normal and pathological conditions.
Basic Res Cardiol 2000;95: 75-83.
9. De Rossi A, Rocha LB and Rossi MA. Application
of fluorescence microscopy on hematoxylin and eosin-stained
sections of healthy and diseased teeth and supporting
structures. J Oral Pathol Med 2007;36: 377-381.
10. El-Beshlawy A, Youssry I, El-Saidi S, El-Accaoui
R, Mansi Y, Maklouf A et al. Pulmonary hypertension
in beta-thalassemia major and the role of L-carnitine
therapy. Pediatr Hematol Oncol 2008;25:734-43.
11. El-Metwally TH, Hamed EA, Ahmad AR, Mohamed
NA. Dyslipidemia, oxidative stress cardiac dysfunction
in children with chronic renal failure: effects
of L-carnitine supplementation. Ann Saudi Med 2003;23:270-7.
12. Esposito LA, Melov S, Panov A, Cottrell BA,
Wallace DC. Mitochondrial disease in mouse results
in increased oxidative stress. Proc Natl Acad Sci
U S A 1999;96:4820-5.
13. Ferrari R, Merli E, Cicchitelli G, Mele D, Fucili
A, Ceconi C. Therapeutic effects of L-carnitine
and propionyl-L-carnitine on cardiovascular diseases:
a review. Ann NY Acad Sci 2003;1033:79-91.
14. Fossati P, Prencipe L. Serum triglycerides determined
colourmetrically with an enzyme that produces hydrogen
peroxide. Clin Chem 1982; 28:2077-80.
15. G_lÅin I. Antioxidant and antiradical
activities of L-carnitine. Life Sci 2006;78:803-11.
16. Gómez-Amores L, Mate A, Miguel-Carrasco
JL, Jiménez L, Jos A, Cameán AM, Revilla
E, Santa-María C, Vázquez CM. L-carnitine
attenuates oxidative stress in hypertensive rats.
J Nutr Biochem 2007;18:533-40.
17. González-Ortiz M, Hernández-González
O, Hernández-Salazar E, Martínez-Abundis
E Effect of oral L-carnitine administration on insulin
sensitivity lipid profile in type 2 diabetes mellitus
patients. Ann Nutr Metab 2008; 52:335-8.
18. Helin HO, Lundin ME, Laakso M, Lundin J, Helin
HJ and Isola J. Virtual microscopy in prostate histopathology:
simultaneous viewing of biopsies stained sequentially
with hematoxylin and eosin, and alpha-methylacyl-coenzyme
A racemase/p63 immunohistochemistry. J Urol 2006;175:
495-499.
19. Heo YR, Lee Y, Cha YS. Carnitine administration
improves lipid metabolism in Streptozotocin-induced
Diabetic rat. Nutr Sci 2002;5:3-8.
20. Khalil FA. Impact of administration of mulberry
juice on blood glucose, lipid profile and oxidative
stress in normal and diabetic rats. The Egyptian
Journal of Biochemistry and molecular biology 2009;27(2):45-62.
21. Li L. End-stage renal disease in China. Kidney
Int 1996; 49:287-301.
22. Mahesh R, Bhuvana S, Hazeena Begum VM. Effect
of Terminalia chebula aqueous extract on oxidative
stress and antioxidant status in the liver and kidney
of young and aged rats. Cell Biochem Funct 2009;
27:358-63.
23. Malone JI, Cuthbertso D, Malon MA and Schocken
DD. Cardio-protective effects of carnitine in streptozotocin-induced
diabetic rats Cardiovascular Diabetology 2006, 5:
1475-2840.
24. Mamoulakis D, Galanakis E, Dionyssopoulou E,
Evangelion A and Sbyrakis S. Carnitine deficiency
in children and adolescents with type 1 diabetes.
Journal of Diabetes and its Complications. 2004;18:271-274.
25. Mansour HH. Effect of L-Carnitine on Endothelial
Dysfunction markers in Diabetic-Irradiated rats.
International Journal of Toxicology and Applied
Pharmacology 2013;3(1): 1-9.
26. McMackin CJ, Widlansky ME, Hamburg NM, Huang
AL, Weller S, Holbrook M, Gokce N, Hagen TM, Keaney
JF Jr, Vita JA. Effect of combined treatment with
alpha-Lipoic acid and acetyl-L-carnitine on vascular
function and blood pressure in patients with coronary
artery disease. J Clin Hypertens 2007;9: 249-255.
27. Miguel-Carrasco JL, Mate A, Monserrat MT, Arias
JL, Aramburu O, Vazquez CM. The role of inflammatory
markers in the cardioprotective effect of L-carnitine
in L-NAME-induced hypertension. Am J Hypertens 2008;21:1231-7.
28. Oka T, Itoi T, Terada N, Nakanishi H, Taguchi
R, Hamaoka K. A. Change in the membranous lipid
composition accelerates lipid peroxidation in young
rat hearts subjected to 2 weeks of hypoxia followed
by hyperoxia. Circ J 2008;72:1359-66.
29. Puddu P, Puddu GM, Cravero E, Rosati M, Muscari
A. The molecular sources of reactive oxygen species
in hypertension. Blood Press 2008;17:70-7.
30. Rajasekar P, Palanisamy N, Anuradha CV. Increase
in nitric oxide and reductions in blood pressure,
protein kinase C beta II and oxidative stress by
L-carnitine: a study in the fructose-fed hypertensive
rat. Clin Exp Hypertens 2007;29:517-30.
31. Rauchova H, Dobesova Z, Drahota Z, Zicha J,
Kunes J. The effect of chronic l-carnitine treatment
on blood pressure and plasma lipids in spontaneously
hypertensive rats ; European Journal of Pharmacology1998;
32. Reitman S, Frankel S. A colorimetric method
for the determination of serum glutamic oxaloacetic
glutamic pyruvic transaminases. Am J Clin Path1957;
28:56-65.
33. Reynolds ES. The use of lead citrate at high
pH as an electron opaque stain in the electron microscopy.
J Cell Biol 1963; 17: 208-212.
34. Rosca MG, Vazquez EJ, Kerner J, Parland W, Chandler
MP, Stanley W et al. Cardiac mitochondria in heart
failure: decrease in respirasomes and oxidative
phosphorylation. Cardiovasc Res. 2008;80:30-9.
35. Ruggenenti P, Cattaneo D, Loriga G, Ledda F,
Motterlini N, Herardi G, Orisio S, Remuzzi G. Ameliorating
Hypertension and Insulin Resistance in Subjects
at Increased Cardiovascular: Risk Effects of Acetyl-l-Carnitine
Therapy. Hypertension 2009; 54: 567-574.
36. Sailaja Devi MM and Das UN. Effect of prostaglandins
against alloxan-induced diabetes mellitus. Prostaglandins
leukotrienes and essential fatty acids. 2005;74:
39-60.
37. Scott JA and King GL. Oxidative stress and antioxidants
treatment in diabetes. Ann NY Acad Sci 1031;2004:204-213.
38. Shibutani S, Takeshita M, Grollman AP. Insertion
of specific bases during DNA synthesis past the
oxidation-damaged base oxod G. Nature 1991;349:431-4.
39. Shiekh GA, Ayub T, Naveed Khan S, Dar A, and
Andrabi KI. Reduced nitrate level in individuals
with hypertension and diabetes. J Cardiovasc Dis
Res 2011; 2(3): 172-176.
40. Tas S, Sarandol E, Ayvalik SZ, Serdar Z and
Dirican M. Vanadyl Sulfate, taurine and combined
vanadyl sulfate and taurine treatments in diabetic
rats: Effects on the oxidative and antioxidative
systems. Archives of Medical Researcl.2007;38:276-283.
41. Trinder P. Determination of glucose in blood
using glucose oxidase with alternative oxygen acceptor.
Ann Clin Biochem1969; 6:24-27.
42. Weber KT, Anversa P, Armstrong PW, Brilla CG,
Burnett JC Jr, Cruickshank JM et al. Remodeling
and reparation of the cardiovascular system. J Am
Coll Cardiol 2001;20:3-16.
43. Würzburg U, Hennrich N, Orth HD, Lang H,
Prellwitz W, Neumeier D, Knedel M, Rick W. Quantitative
determination of creatine kinase isoenzyme catalytic
concentrations in serum using immunological methods.
J Clin Chem Clin Bioch1977; 15:131-7.
44. Yao H, Lin P, Chang Y, Chen G, Chiang M, Chang
L. Nuo Y, Tsai H, and The T. Effect of taurine supplementation
on cytochrome P 450 2E, and oxidative stress in
the liver and kidneys of rats with streptozotocin-induced
diabetes; Food and chemical toxicology 2009; 47:1703-1709.
45. Zambrano S, Blanca AJ, Ruiz-Armenta MV, Miguel-Carrasco
JL, Revilla E, Santa-María C, Mate A, Vázquez
CM. The renoprotective effect of L-carnitine in
hypertensive rats is mediated by modulation of oxidative
stress-related gene expression. European Journal
of Nutrition 2013; 52: 1649-1659.
46. Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial
ROS-induced ROS release: an update and review. Biochim
Biophys Acta 2006;1757:509-17.
|
