|
Paclitaxel-Carboplatin
versus bevacizumab Paclitaxel-Carboplatin for
treatment of Non-Small-Cell Lung Cancer
......................................................................................................................................................................
Waleed Hammam
(1)
Yasser M Saleh
(2)
(1) Department of Clinical Oncology , Kasr Eleini,
Cairo University.
(2) Department of Clinical Oncology and Nuclear
Medicine, Almansoura University
Correspondence:
Dr Waleed Hammam Mosa,
Department of Clinical Oncology
Kasr Eleini,
Cairo University
Egypt
Tel: 00966544687735
Email: oncology1.ryd@sghgroup.net
|
ABSTRACT
Background: Lung cancer is considered
as the leading cause attributed to cancer
related deaths and approximately 85% of
lung cancer patients have non-small-cell
lung cancer (NSCLC) and Vascular endothelial
growth factor (VEGF) is used to play the
major role in regulation of angiogenesis
in malignancies.
Aim: The aim of this study was to compare
chemotherapy alone in comparison with addition
of anti -vegf (bevacizumab) to chemotherapy
and assessment of response rate , progression
free survival, overall survival in patients
diagnosed with non- squamous non small cell
lung cancer in Saudi German hospitals in
the period between March 2013 and February
2016.
Patients and methods: This study
was held between March 2013 and February
2016 in Saudi German hospitals when we performed
a randomized study in which 40 patients
with recurrent or advanced non-small-cell
lung cancer (stage IIIB or IV) received
paclitaxel and carboplatin (paclitaxel-carboplatin
arm) (20 patients) paclitaxel and carboplatin
in addition to bevacizumab (paclitaxel-carboplatin-bevacizumab
arm) (20 patients).
Results: The median overall survival
was 15.5 months in the paclitaxel-carboplatin-bevacizumab
arm as compared with 10.5 months in the
paclitaxel-carboplatin arm ( P=0.002) and
the median progression-free survival was
also significantly improved in the paclitaxel-carboplatin-bevacizumab
arm reaching (8.4 months versus 5.9 in the
paclitaxel-carboplatin arm) for a hazard
ratio for disease progression of 0.67 (95%
CI, 0.57 to 0.77; P<0.001) and the addition
of bevacizumab to paclitaxel and carboplatin
improved the response rate as (25 %) in
the paclitaxel-carboplatin arm had a response
versus (65%) in the paclitaxel-carboplatin-bevacizumab
arm (P<0.001) and the rates of hypertension,
bleeding, thrombocytopenia, neutropenia,
febrile neutropenia, proteinuria were significantly
higher in the paclitaxel-carboplatin-bevacizumab
arm than in the paclitaxel-carboplatin arm.
(P<0.05).Conclusion: The addition of
bevacizumab to the chemotherapy added a
significant value to the patients with non
squamous NSCLC in terms of response rate,
progression free survival and overall survival
however with significant side effects.
Key words: Lung cancer; Bevacizumab;
Vascular endothelial growth factor
|
Lung cancer is considered as
the leading cause attributed to cancer related
deaths and approximately 85% of lung cancer patients
have non-small-cell lung cancer and there is global
rise of lung cancer incidence with overall 5 years
survival less than 15% .(1) Tumorogenesis is considered
as a multistep process that depends on transformation
from normal bronchial epithelium to overt lung
cancer then continued accumulation of the genetic
abnormalities influences the cancer invasion and
development of metastases and resistance to the
cancer treatment and that can take place throughout
chromosomal instability mechanisms.(2) Several
aetiological factors have been accused in NSCLC
including cigarette smoking , exposure to radon
, asbestos and genetic susceptibility.(3) NSCLC
has 3 major histological subtypes, adenocarcinoma,
large cell carcinoma and squamous cell carcinoma.(4)
In early stages stage I and II, and selected cases
of stage III, surgery is the corner stone of management
followed by adjuvant chemotherapy but in late
stages, the unresectable stage III the treatment
is chemoradiation and in stage IV the treatment
is double agent chemotherapy with or without biological
target therapy .(5) After their growth within
the bronchial wall and or the lung parenchyma
primary lung malignant tumors invade the regional
hilar and mediastinal lymph nodes through lymphatics
then through the blood vessels to distant organs
such as brain, liver and bone.(6) Biopsy can be
performed through several methods including CT
guided biopsy or bronchoscopic biopsy and even
through thoracoscopy or thoracotomy and Positron
Emission Tomography (PET scan) is a corner stone
in staging and further assessment during treatment
and follow up.(7)
Angiogenesis is a landmark for cancer in which
there is an angiogenic switch from perturbation
in the balance that normally exists between inducers
and inhibitors which are produced by both tumor
and host cells that lead to a high micro vessels
density with overexpression of VEGF which is associated
with poor outcome in NSCLC.(8) Vascular endothelial
growth factor (VEGF) is used to play the major
rule in regulation of angiogenesis in malignancies
Increased VEGF expression in non-small-cell lung
cancers is associated with increased risks of
local recurrences, metastases, and deaths.(9)
Preclinical studies have shown that a monoclonal
antibody against VEGF ( bevacizumab-avastin) can
inhibit the growth of human malignant tumor cells
(10).new target therapy agents are needed to overcome
the intrinsic or acquired resistance limiting
the efficacy of the common anti-tumoral agents
(11).
This study was held in Saudi German hospitals
and is a randomized study including patients with
advanced non-small-cell lung cancer with no prior
chemotherapy administration compared to paclitaxel
and carboplatin protocol versus paclitaxel and
carboplatin plus bevacizumab protocol with bevacizumab
dose 15 mg /kg of body weight intravenously every
3 weeks.(12)
This study was held between March
2013 and February 2016 in Saudi German hospitals
as we performed a randomized study in which 40
patients with recurrent or advanced non-small-cell
lung cancer (stage IIIB patient -pleural effusion-
or IV) received paclitaxel and carboplatin (paclitaxel-carboplatin
arm) (20 patients) or paclitaxel and carboplatin
in addition to bevacizumab (paclitaxel-carboplatin-bevacizumab
arm) (20 patients). Inclusion criteria were patients
with an ECOG performance status of 0-2, and adequate
hematologic, hepatic, and renal function and to
be histopathologically proved newly diagnosed
stage IIIB ( pleural effusion) or stage IV non-squamous
NSCLC or recurrent NSCLC with no prior chemotherapy.
Exclusion criteria were histologic evidence of
squamous-cell cancer or central nervous system
(CNS) metastases, pregnancy or lactation, significant
cardiovascular disease and uncontrolled hypertension.
The primary end point was overall survival. In
our study patients were randomly assigned to receive
paclitaxel at a dose of 175 mg/m2 and carboplatin
at a dose of area under the curve (AUC) 6 administered
intravenously on day 1 (arm 1), or paclitaxel
at a dose of 175 mg/m2 and carboplatin at a dose
of area under the curve (AUC) 6 administered in
addition to bevacizumab at a dose of 15 mg /kg
given intravenously on day 1 and chemotherapy
was repeated every 21 days for a total of six
cycles unless there was disease progression or
marked intolerable toxicity. Patients in the paclitaxel-carboplatin-bevacizumab
group continued to receive bevacizumab alone every
3 weeks unless there was disease progression or
marked intolerable toxicity.
Afterwards the baseline evaluation assessment
took place every 9 weeks by PET scan assessment.
Survival was measured as the period from randomization
to death , and progression-free survival as the
period from randomization to disease progression
or death. Event-time distributions were estimated
by the Kaplan-Meier method and estimated P values
were two-sided and CIs were at the 95% level.
The two
groups were well balanced regarding baseline characteristics
and the median number of cycles of therapy was
five in the paclitaxel-carboplatin arm and seven
in the paclitaxel-carboplatin-bevacizumab arm.
The median overall survival was 15.5 months in
the paclitaxel-carboplatin-bevacizumab arm as
compared with 10.5 months in the paclitaxel-carboplatin
arm ( P=0.002). Survival rates were 55% in the
paclitaxel-carboplatin-bevacizumab arm as compared
with 45% in the paclitaxel-carboplatin arm at
1 year and 27% as compared with 17% respectively
at 2 years.
The median progression-free survival was also
significantly improved in the paclitaxel-carboplatin-bevacizumab
arm reaching ( 8.4 months versus 5.9 in the paclitaxel-carboplatin
arm) for a hazard ratio for disease progression
of 0.67 (95% CI, 0.57 to 0.77; P<0.001).
The addition of bevacizumab to paclitaxel and
carboplatin improved the response rate as (25
%) in the paclitaxel-carboplatin arm had a response
versus (65%) in the paclitaxel-carboplatin-bevacizumab
arm (P<0.001). (Table 1)
Table 1:
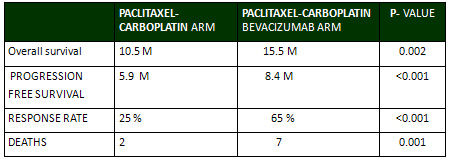
The rates of hypertension, bleeding, thrombocytopenia,
neutropenia, febrile neutropenia, proteinuria
were significantly higher in the paclitaxel-carboplatin-bevacizumab
arm than in the paclitaxel-carboplatin arm (P<0.05).
There were 9 deaths related to toxic effects of
the treatment. Two deaths (from gastrointestinal
hemorrhage and febrile neutropenia) occurred in
patients in the paclitaxel-carboplatin arm and
7 occurred in the paclitaxel-carboplatin-bevacizumab
arm; the difference between the groups was significant
(P=0.001)(Table 1). Of the 7 deaths in the paclitaxel-carboplatin-bevacizumab
group, 4 were due to pulmonary hemorrhage, and
3 due to febrile neutropenia.
In our study the addition of
bevacizumab to chemotherapy regimen improved overall
survival, progression-free survival and response
rate in patients with advanced NSCLC.
Villett et al. stated that bevacizumab increases
delivery of the drug to the tumor and the marvelous
significant improvement in the response rate in
this study and in previous randomized controlled
studies of chemotherapy with addition of bevacizumab
supports the data that bevacizumab improves overall
survival, progression-free survival and response
rate in patients with advanced NSCLC.(13)
Jubb AM et al stated in their study the use of
bevacizumab in combination with platinum based
chemotherapy in treatment of metastatic lung cancer
stage IV patients resulted in improvement of response
rate and progression free survival which is matching
with the results revealed in our study.(14)
Among the 9 other deaths considered to be related
to treatment in our study, 5 were due to haemorrhage
either pulmonary or gastrointestinal and 4 were
due to complications of febrile neutropenia and
although neutropenia has not been associated with
bevacizumab, however 3 patients in the paclitaxel-carboplatin-bevacizumab
group had grade 5 febrile neutropenia. Other studies
had as that reported by Giantonio BJ et al and
by Jubb AM et al revealed increased rates of neutropenia
when bevacizumab was combined with chemotherapy.(14,
15 )
The hypertension, and proteinuria in our study
are due to bevacizumab. They were manageable and
did not need a permanent stop of bevacizumab.
This is matched with other literature as reported
by Jubb AM et al and Kozloff M. et al in the study
of treatment effects and side effects of bevacizumab
(15,16).
The addition of bevacizumab to
the chemotherapy added a significant value to
the patients with non squamous NSCLC in terms
of response rate, progression free survival and
overall survival however with significant side
effects.
1- Jemal A, Murray T, Ward E,
et al. Cancer statistics, 2010. CA Cancer J Clin
2005;55:10-30. [Erratum, CA Cancer J Clin 2005;55:259.]
2- The hallmarks of cancer. Cell 2008;100:57-70
3- Ferrara N. The role of vascular endothelial
growth factor in pathological angiogenesis ;N
Eng J Med 2012 444:98-4.
4- Schiller JH, Harrington D, Belani CP, et al.
Comparison of four chemotherapy regimens for advanced
non-small-cell lung cancer. N Engl J Med 2002;346:92-8.
5- Seto T, Higashiyama M, Funai H, et al. Prognostic
value of expression of vascular endothelial growth
factor and its flt-1 and KDR receptors in stage
I non-small-cell lung cancer. Lung Cancer 2006;53:91-6.
6- Ferrara N, Gerber HP, Le Couter J. The biology
of VEGF and its receptors. Nat Med 2014;9:669-76.
7- Brown LF, Berse B, Jackman RW, et al. Expression
of vascular permeability factor (vascular endothelial
growth factor) and its receptors in breast cancer.
Hum Pathol 2007;26:86-91.
8-Hurwitz H, Fehrenbacher L, Novotny W, et al.
Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N
Engl J Med 2004;350: 2335-42.
9-Johnson DH, Fehrenbacher L, Novotny WF, et al.
Randomized phase II trial comparing bevacizumab
plus carboplatin and paclitaxel with carboplatin
and paclitaxel alone in previously untreated locally
advanced or metastatic non-small-cell lung cancer.
J Clin Oncol 2004;22:2184-91.
10- James K, Eisenhauer E, Christian M, et al.
Measuring response in solid tumors: unidimensional
versus bidimensional measurement. J Natl Cancer
Inst 2009;91: 523-8.
11- Jain RK. Normalizing tumor vasculature with
anti-angiogenic therapy: a new paradigm for combination
therapy. Nat Med 2010;7:987-9.
12- Miller KD, Wang W, Gralow J, al. A randomized
phase III trial of paclitaxel versus paclitaxel
plus bevacizumab as first-line therapy for locally
recurrent or metastatic breast cancer: a trial
coordinated by the Eastern Cooperative Oncology
Group (E2100). Breast Cancer Res Treat 2005;94:
Suppl 1:S6. abstract.
13- Villett CG, Boucher Y, di Tomaso E, et al.
Direct evidence that the VEGF-specific antibody
bevacizumab has antivascular effects in human
rectal cancer. Nat Med 2004;10:145-7. [Erratum,
Nat Med 2004;10:649.]
14- Giantonio BJ, Catalano
PJ, Meropol NJ, et al. High-dose bevacizumab improves
survival when combined with FOLFOX4 in previously
treated advanced colorectal cancer: results from
the Eastern Cooperative Oncology Group (ECOG)
study E3200. J Clin Oncol 2009;23:Suppl:16S. abstract.
15- Jubb AM, Hurwitz HI, Bai W, et al. Impact
of vascular endothelial growth factor-A expression,
thrombospondin-2 expression, and microvessel density
on the treatment effect of bevacizumab in metastatic
lung cancer. J Clin Oncol 2006;24:217-27.
16 -Kozloff M, Cohn A, Christiansen N, et al.
Safety of bevacizumab among patients receiving
first-line chemotherapy for metastatic colorectal
cancer: preliminary results from a larger registry
in the U.S. J Clin Oncol 2005;23:Suppl:16S. abstract.
|
