|
Resistin, an adipokine,
its relation to inflammation in Systemic Lupus
Erythematosus and Rheumatoid Arthritis
......................................................................................................................................................................
Mohamed Hassan Hammad (1)
Sherif Nasef (2)
Mohamed Yousry Elsammak (3)
Poovathumkadavi Mammunji Abduljaleel (4)
Douaa Musalam (5)
Mohieldin M Ahmed (6)
Ibrahim Osman (7)
(1) Mohamed Hassan Hammad (MD), Assistant consultant
Physical Medicine and Rehabilitation
at King Fahad Specialist Hospital Dammam (KFSHD),
Saudi Arabia.
(2) Sherif Nasef (MD), Consultant rheumatology
at KFSHD, Saudi Arabia.
(3) Mohamed Yousry Elsammak (MD), Consultant Immunology
Lab at KFSHD, Saudi Arabia.
(4) Poovathumkadavi Mammunji Abduljaleel (MD),
Consultant Radiology at KFSHD, Saudi Arabia.
(5) Douaa M Mosalem (MD): Specialist Physical
Medicine and rheumatology, Ministry of Health,
Kuwait.
(6) Mohieldin M Ahmed (MD): Consultant Physical
Medicine and rheumatology, Ministry of Health,
Kuwait.
(7) Ibrahim Osman: Statistician at KFSHD, Saudi
Arabia.
Correspondence:
Dr. Douaa M Mosalem (MD): Specialist Physical
Medicine and Rehabilitation,
Ministry of Health, Kuwait
Email: dr.douaa@hotmail.com
|
ABSTRACT
Objective: To determine the difference
in serum resistin levels in Systemic lupus
Erythematosus (SLE) and Rheumatoid arthritis
(RA) patients compared to a control group.
Also, to find the relationship between serum
resistin levels and disease activity in
SLE and RA patients.
Subjects and Methods: This study included
three groups of 30 SLE patients, 30 RA patients
and 30 apparent healthy volunteers. All
patients were subjected to full history
taking, clinical examination, laboratory
assessment (ESR, CRP, renal function, urine
examination, lipid profile, RF, ANA, anti-dsDNA,
ACPA, C3 and C4), X-ray both hands for RA
patients for both SLE and RA patients, assessment
of disease activity according to SLEDAI
for SLE patients and according to DAS 28
score for RA patients and assessment of
radiological damage for RA patients using
Larsen score. Serum samples from all patients
and controls were tested for serum resistin
levels.
Results: The mean of serum resistin
levels in SLE (2.86±0.02 ng/ml) and
RA (3.002±0.06 ng/ml) were insignificantly
higher than controls (2.14± 0.08ng/ml)
(p=0.233 and p=0.07 respectively) . There
was no significant difference between serum
resistin levels between SLE and RA patients
(p=0.586). There were insignificant correlations
between disease duration and all laboratory
parameters compared to serum resistin levels
in SLE and RA (p>0.05) but the platelets
had an inverse significant correlation with
serum resistin levels in SLE (p<0.022).
There was insignificant correlation between
serum levels of resistin and SLEDAI in SLE
(p=0.180). Moreover, there was insignificant
correlation between and DAS 28 and Larsen
score compared to serum resistin levels
in RA (p=0.207, p=0.735, respectively).
Conclusion: Serum resistin levels
did not correlate with clinical or laboratory
markers except platelet counts in SLE and
or RA cases, although it is a higher level
in these diseases compared to the controls.
Key words: Resistin, SLE and RA.
|
Systemic Lupus Erythematosus
(SLE) is a disease characterized by systemic inflammation
with the property of affecting several organs
throughout the body (1). Rheumatoid arthritis
(RA) is a systemic autoimmune inflammatory disorder
of unknown etiology that primarily affects the
synovial lining of the diarthrodial joints. It
is characterized by symmetric, erosive synovitis
and in some cases extra-articular involvement
(2); most patients experience a chronic fluctuating
course of disease that, despite therapy, may result
in progressive joint destruction, deformity, disability,
and even premature death (3).
Resistin is a low-molecular-weight adipokine also
known as the adipocyte specific secretory factor
that was independently identified by three groups
(4). It is an adipocyte secreted hormone belonging
to a cysteine-rich protein family. It is expressed
in white adipose tissues in rodents and has also
been found in several other tissues in humans.
Insulin, glucose, many cytokines and anti-diabetic
thiazolidinediones are regulators of resistin
gene expression (5).
The role of resistin in humans has not been fully
established (6). It was first proposed to be involved
in insulin resistance and type 2 diabetes, but
later, it was found to be relevant to inflammation
and inflammation-related diseases like atherosclerosis
and arthritis (5). There was evidence that resistin
has proinflammatory properties, is abundant in
inflammatory diseases e.g., RA and Crohn's disease;
and is also associated with inflammatory markers
in several different populations (7, 8).
Resistin was found accumulated in inflamed joints
of patients with RA and had the capacity to induce
arthritis in mice. In humans, resistin is expressed
in inflammatory cells, leukocytes, and macrophages
and has the potency of inducing production of
interleukin -6 and tumor necrosis factor-alpha
(9, 10).
The aim of this work is to determine
the level of resistin in the serum of patients
with SLE and RA. The aim extends to examine the
relationship and possible associations between
the serum resistin levels and different markers
of disease activity, inflammation, renal function
and lipids with RA and SLE patients.
Thirty
patients fulfilling at least four of the updated
American College of Rheumatology (ACR) revised
criteria for the classification of systemic lupus
erythematosus (SLE) (11), thirty patients fulfilling
at least four of the 1987 Revised ACR Criteria
for the classification of rheumatoid arthritis
(RA (12) and 30 apparent healthy volunteers matched
for age and sex with the SLE and RA were enrolled
in this study.
These patients were recruited
from the in-patients and out-patients' clinic
of the Rheumatology, Rehabilitation and Physical
Medicine Department of King Fahad Specialist Hospital
Dammam Saudi Arabia. Informed consent was obtained
from all participants, and the study was approved
by the IRB committee of King Fahad Specialist
Hospital Dammam.
Patients with the following conditions were excluded
from the study including pre-existing diseases
causing nephritis, evidence of malignancy, concurrent
infection and diabetes in patients and controls.
All the patients and controls were subjected to
complete history taking as well as thorough clinical
examination. Assessment of disease activity of
SLE was done using Systemic Lupus Erythematosus
Disease Activity Index (SLEDAI) (13). Grading
of SLE disease activity (SLEDAI) includes Mild
activity: 1-10, Moderate activity: 11-20, Severe
activity: 21-45, Very severe activity: >45
(13).
Assessment of the Disease Activity Score 28 (DAS28)
was done in patients with RA (14). The rheumatoid
Disease Activity Score 28 (DAS28) was determined
from scores as follows: Remission: DAS 28 <
2.6, Low disease activity: DAS 28 >2.6 <
3.2, Moderate disease activity: DAS 28 > 3.2
and < 5.1,
High disease activity: DAS 28 > 5.1. DAS 28
= [0.56 ×  (tender
28) + 0.28 × (tender
28) + 0.28 ×  (swollen
28) + 0.70 × Ln (ESR)] 1.08 + 0.16 (14). (swollen
28) + 0.70 × Ln (ESR)] 1.08 + 0.16 (14).
All patients were subjected to the following lab
tests as indicated by their disease, using standard
laboratory techniques Erythrocyte Sedimentation
Rate [ESR] by Westergren method, C-reactive protein
(CRP) by latex agglutination slide test, serum
creatinine and blood urea, complete urine analysis,
complete blood count, C3 & C4 by using a standard
nephelometric technique, ANA by using a standard
immune-fluorescence technique, Anti double stranded
DNA by using ELISA testing and Plasma lipoproteins
by using a standard colorimetric reaction.
Serum resistin levels were determined in patients
and controls by using a quantitative sandwich
Enzyme-Linked Immunosorbent Assay (ELISA). 2 ml
venous blood samples are taken after a one-night
fast, and serum from these samples will be stored
at -70°C until the time of analyses according
to a standard ELISA technique using a Quantikine
ELISA kit for Human Resistin supplied by R&D
Systems USA.
Plain X-ray of both hands and wrists, postero-anterior
views, were done for all RA patients. Radiographic
damage specific for RA is evaluated by Larsen
method (LS) for each of the patients (15).
Statistical analysis was performed using
an IBM computer utilizing Statistical Package
for Social Science (SPSS) program version 16.
Continuous data were expressed in the form of
mean ±SD while categorical data were expressed
in the form of count and percent. The difference
between the two groups was analyzed via student's
t-test. One-way analysis of variance (ANOVA) was
used to compare more than two groups. Spearman's
correlation coefficient (r) was used to assess
the degree of association between 2 continuous
variables.
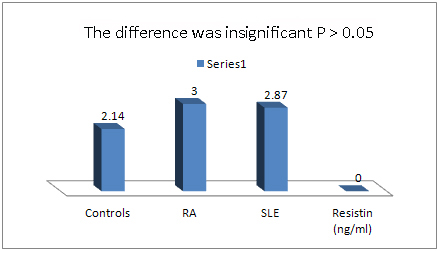
The mean of serum resistin levels
in SLE (2.866 ng/ml) and RA (3.002 ng/ml) were
insignificantly higher than controls (2.14 ng/ml)
(p=.233 and p=.233 respectively). There was no
significant difference between serum levels of
resistin between SLE and RA parients (p=0.098).
There were insignificant correlations between
disease duration and all laboratory parameters
compared to serum resistin levels in SLE and RA
(p>0.05) but the platelets had an inverse significant
correlation with serum resistin levels in SLE
(p< 0.022). There was insignificant correlation
between serum levels of resistin and SLEDAI in
SLE (p=0.180).
There was insignificant correlation between DAS
28 and Larsen score compared to serum levels of
resistin in RA (p=0.207, p=0.735, respectively)
(p>0.05). The demographic characteristics,
clinical and laboratory findings of all the studied
groups are demonstrated in Table 1. Table 2 shows
correlations of serum resistin levels with clinical
and laboratory data in SLE and RA patients. While,
Table 3 and Figure 1 reveal correlation of serum
resistin levels in SLE, RA patients and controls.
Table 1: Demographic characteristics, clinical
and Laboratory data in SLE and RA patients and
control (ANOVA test)
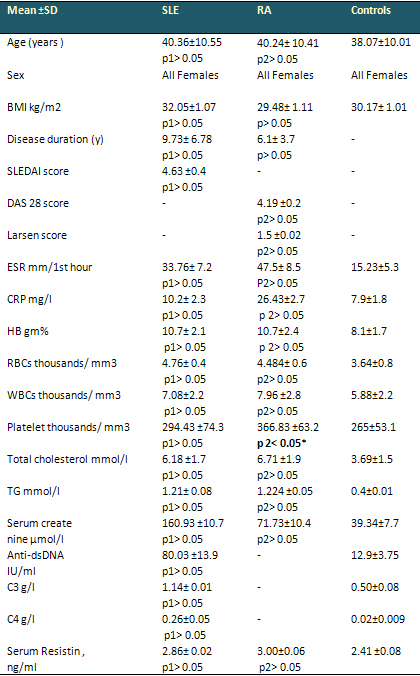
• Relation between two groups of SLE and
RA patients and control group,
• p 1= between SLE patients and control,
p 2= between RA patients and controls.
• Non-Significant (NS) p > 0.05; Significant
(S) * p < 0.05
Table 2: Correlations of serum resistin levels
with clinical and laboratory data in SLE and RA
patients
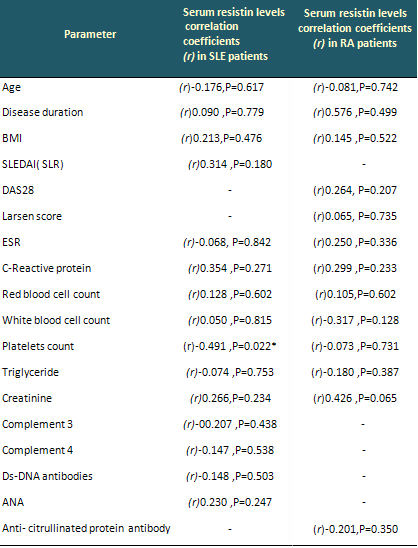
• All clinical and laboratory parameters
had insignificant correlations with serum resistin
levels. Non-Significant (NS) (p > 0.05).
Table 3: Correlation of Serum Resistin levels
in SLE, RA patients and control (ANOVA test)
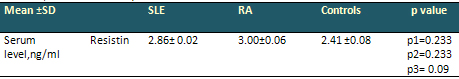
• Relation between two groups of SLE and
RA patients and control group,
• p 1= between SLE patients and control,
p 2= between RA patients and control, p 3= between
SLE patients and RA. Non-Significant (NS) p >
0.05.
Figure 1: Mean of Serum Resistin Levels in SLE,
RA and Control groups
The immune system requires a
proper energy balance for its physiological functions.
In the past years, an important connection has
been evidenced between that system and metabolism,
with the identification of obesity as a predisposing
factor for the development of several disorders,
such as some immune-mediated diseases. The adipose
tissue is not inert, and has been considered an
organ with immune and neuroendocrine functions.
That tissue produces several mediators, such as
resistin, tumor necrosis factor alpha (TNF-a),
interleukin 6 (IL-6), interleukin 1 (IL-1), chemokine
ligand 2 (CCL2), plasminogen activator inhibitor
type 1, and complement components, all participating
in the innate immune response as pro-inflammatory
mediators (16).
Macrophages are components of adipose tissue and
actively participate in its activities. Furthermore,
cross-talk between lymphocytes and adipocytes
can lead to immune regulation. Adipose tissue
produces and releases a variety of pro-inflammatory
and anti-inflammatory factors, including resistin,
the adipocytokines leptin, adiponectin, and visfatin,
as well as cytokines and chemokines, such as TNF-a,
IL-6, monocyte chemo-attractant protein-1, and
others. Reduced leptin levels might predispose
to increased susceptibility to infection caused
by reduced T-cell responses in malnourished individuals
(17).
Resistin, a novel adipocyte-secreted hormone,
has gained attention for its involvement in insulin
resistance in obesity and diabetes mellitus. Several
groups have reported a close relationship between
resistin and inflammation. Resistin increases
the production of pro-inflammatory cytokines TNF-a
and interleukin (IL)-12, both of which are important
for T cell development (18).
In the current study, the mean serum level of
resistin was highest in RA patients although there
were insignificant differences of its level between
SLE patients and controls (p=0.233), RA patients
and controls (p=0.07) as well as SLE and RA patients
(p=0.586). There is no agreement over the concentrations
and function of resistin in SLE, because of a
limited number of studies and their inconsistent
results.
Data demonstrated by several authors were in agreement
with our results. Almehed et al. found that serum
resistin levels in controls were similar to those
of SLE patients (1). Chung et al. have assessed
the concentrations of resistin, visfatin, leptin
and adiponectin in 109 patients with SLE. They
did not find statistically significant differences
in resistin concentrations among SLE patients
and control subjects (p=0.41) (19). Otero et al.
(20) and Forsblad et al. (21) also found no difference
in resistin concentration between RA patients
and healthy controls. Yoshino et al. reported
that there were no statistically significant differences
in serum resistin levels between the RA patients
(22).
Moreover, Yee et al. (23), Heilbronn et al. (24)
and Iqbal et al. (25) showed no significant correlation
between BMI and resistin levels in normal individuals.
Senolt et al. (26) and Canruc et al. (27) found
no significant correlation was found between BMI
and serum resistin levels in RA patients. Bokarewa
et al. did not find a relation between serum resistin
levels and disease duration in RA patients. No
significant correlations were found between serum
resistin levels of SLE or RA patients and their
disease duration (28). Bokarewa et al. found no
significant difference of resistin levels between
RA patients and healthy controls. Resistin levels
in blood were neither related to the duration
of RA, age of the patients, nor to circulating
C-reactive protein levels or white blood cell
counts (28).
In addition, Canruc et al. (27) and Kassem et
al. (29) found no significant correlation was
found between BMI and serum resistin levels in
SLE or RA patients. Canruc et al. did not find
a relation between serum resistin levels and disease
duration in RA patients. They found no significant
correlations between ESR or CRP and serum resistin
levels in RA patients (27). Bokarewa et al. showed
resistin levels in blood were not related to circulating
C-reactive protein level in RA patients (28).
Bokarewa et al. (28), Canruc et al. (27) found
no significant correlation between serum resistin
levels and white blood cell count in RA patients.
Elshishtawy et al. found insignificant correlation
between serum resistin levels and SLEDAI (p>0.05)
(30).
On the contrary, Elshishtawy et al. found a highly
significant difference in the serum resistin levels
of SLE patients compared to the control group
(p<0.0001) (30). Migita K et al. (31) found
serum resistin levels to be significantly higher
in RA patients compared to the control subjects
(P= 0.0005) (1). Also, Yoshino et al. (21) found
significant correlation between serum levels of
resistin and BMI (1). Zhang et al. (32) and Yannakoulia
et al. (33) reported about correlation of resistin
levels with BMI in normal individuals where resistin
levels correlated significantly with adiposity
in obese individuals.
In contrast to our result, Migita et al. (31),
Senolt et al. (26) and Kassem et al. (29) found
statistically significant correlations between
resistin levels in the serum of RA patients and
ESR and CRP. However, Senolt et al. (26) found
a positive association between serum resistin
level and disease duration in patients with RA
(1). Gonzalez et al. found a highly significant
association between the platelet count and resistin
levels in RA patients (34). In our study, we find
any significant negative correlation between serum
resistin levels with the platelet count in SLE
patients (r = -0.491,p=0.022), but Elshishtawy
et al. had a statistically significant positive
correlation between platelet count and serum resistin
levels in SLE patients (30).
Almehed et al. found a relationship between serum
resistin levels and the severity of inflammation,
bone mass density (BMD) and renal function in
SLE patients (1). They stated that the association
between resistin, ESR, and complement 3 (C3) levels,
observed in their study, may reflect disease activity
(1).
However, Senolt et al. found a positive correlation
between serum resistin levels and disease activity
based on DAS 28 in patients with RA. Forsblad
et al. (21), Kassem et al. (29) and Rho et al.
(35) found a significant positive correlation
between serum resistin levels and Larsen score
for radiological joint damage in RA patients (p<
0.05). In one study, the authors found a relationship
between serum resistin levels and the severity
of inflammation and renal function in SLE patients
(1).
In our study, explanation
of serum resistin levels did not correlate with
clinical or laboratory markers except platelet
counts; it could be due to difference in disease
activities, age, BMI, disease duration in SLE
and RA patients. Also, this can be explained by
the fact that serum resistin may not have a main
role in the pathogenesis of these diseases, but
other mediators may have a main role in the pathogenesis
of SLE and or RA patients.
We conclude that serum resistin levels did not correlate
with clinical or laboratory markers except platelet
counts in SLE and or RA cases, although it has a
higher level in these diseases compared to the controls.
In explanation of our results, it could be difference
in disease activities, age, BMI or disease duration
in SLE and RA patients. Moreover, resistin may not
have a main role in the pathogenesis of SLE and
or RA patients. We recommended that further longitudinal
studies including a greater number of patients are
required and further comparative studies are required.
1. Almehed K, d'Elia HF, Bokarewa M, et al. Role
of resistin as a marker of inflammation in systemic
lupus erythematosus. Arthritis Res Ther. 2008; 10(1):
15.
2. Harris ED. Rheumatoid arthritis: pathophysiology
and implications for therapy. N Engl J Med1990 ;322:1277-89
3. Hochberg MC (1981). Adult and juvenile rheumatoid
arthritis: current epidemiologic concepts. Epidemiol
Rev 1981; 3:27-44.
4. Holcomb IN, Kabakoff RC, Chan B, et al. FIZZ1,
a novel cysteine-rich secreted protein associated
with pulmonary inflammation, defines a new gene
family. EMBO J 2000; 19: 4046-4055.
5. Shanshan Pang and Yingying Le. Role of Resistin
in Inflammation and Inflammation-Related Diseases.
Cellular & Molecular Immunology 2006; 3(1):29-34.
6. Steppan CM, Bailey ST, Bhat S, et al. The hormone
resistin links obesity to diabetes. Nature 2001;
409:307-312.
7. Migita K, Miyashita T, Maeda Y, et al. Toll-like
receptor expression in lupus peripheral blood mononuclear
cells. J Rheumatol 2007; 34:493.
8. Karmiris K, Koutroubakis IE, Xidakis C, et al.
Circulating levels of leptin, adiponectin, resistin,
and ghrelin in inflammatory bowel disease. Inflamm
Bowel Dis 2006; 12:100-105.
9. Patel L, Buckels AC, Kinghorn IJ, et al. Resistin
is expressed in human macrophages and directly regulated
by PPAR gamma activators. Biochem Biophys Res Commun
2003; 300:472-476.
10. Bokarewa M, Nagaev I, Dahlberg L, et al. Resistin,
an adipokine with potent proinflammatory properties.
J Immunol 2005; 174:5789-5795.
11. Hochberg MC. Updating of the American college
of Rheumatology (ACR) revised criteria for the classification.
Arthritis Rheum1997; 40:1725.
12. Arnett FC, Edworthy SM, and Bloch DA: complete
et al. The American Rheumatism Association 1987
revised criteria for the classification of rheumatoid
arthritis. Arthritis Rheum1988; 31(3):315-24.
13. Bombardier C, Gladman DD, Urowitz MB, et al.
Derivation of the SLEDAI. A disease activity index
for lupus patients. The Committee on Prognosis Studies
in SLE. Arthritis Rheum1992; 35:630-640.
14. Prevoo ML, van 't Hof MA, Kuper HH et al. Modified
disease activity scores that include twenty-eight-joint
counts. Development and validation in a prospective
longitudinal study of patients with rheumatoid arthritis.
Arthritis Rheum1995; 38:44-48.
15. Larsen A. How to apply Larsen score in evaluating
radiographs of rheumatoid arthritis in long-term
studies. J Rheumatol1995; 22:1974-1975.
16. Vitalina de Souza Barbosa, Jozelia Rego and
Nilzio Antonio da Silva. Possible role of adipokines
in systemic lupus erythematosus and rheumatoid arthritis.
Rev Bras Reumatol 2012; 52(2):271-287.
17. Fantuzzi G. "Adipose tissue, adipokines,
and inflammation," Journal of Allergy and Clinical
Immunology2005; vol.115, no. 5, pp. 911-920.
18. Son YM, Ahn SM, Jang MS, et al. Immunomodulatory
effect of resistin in human dendritic cells stimulated
with lipoteichoic acid from Staphylococcus aureus.
Biochem Biophys Res Commun 2008; 376:599-604.
19. Chung Cecilia P, Long Ashley G, Solus Joseph
F, et al. Adipocytokines in Systemic Lupus Erythematosus:
Relationship to Inflammation, Insulin Resistance
and Coronary Atherosclerosis. Lupus. 2009; 18(9):
799-806.
20. Otero M, Lago R, Gomez R, et al. Changes in
plasma levels of fat-derived hormones adiponectin,
leptin, resistin and visfatin in patients with rheumatoid
arthritis. Ann Rheum Dis 2006; 65(9):1198-201.
21. Forsblad d'Elia H, Pullerits R, Carlsten H,
et al. Resistin in serum is associated with higher
levels of IL-1Ra in post-menopausal women with rheumatoid
arthritis," Rheumatology 2008 ; vol. 47, no.
7, pp. 1082-1087.
22. Yoshino T, Kusunoki N, Tanaka N et al. Elevated
Serum Levels of Resistin, Leptin, and Adiponectin
are Associated with C-reactive Protein and also
Other Clinical Conditions in Rheumatoid Arthritis.
Intern Med 2011; 50: 269-275.
23. Yee S, Minn AH, Patterson NB et al. Resistin
is expressed in pancreatic islets. Biochem Biophys
Res Commun 2003; 17; 310(2):641-5.
24. Heilbronn LK, Rood J, Janderova L, et al. Relationship
between serum resistin concentrations and insulin
resistance in nonobese, obese, and obese diabetic
subjects. J Clin Endocrinol Metab 2004; 89 (4):
1844-8.
25. Iqbal I, Seshadri B, Sterni L et al. Serum resistin
is not associated with obesity or insulin resistance
in humans. European Review for Medical and Pharmacological
Sciences 2005; 9: 161-165.
26. Senolt L, Housa D, Vernerova Z, et al. Resistin
in rheumatoid arthritis synovial tissue, synovial
fluid and serum," Annals of the Rheumatic Diseases
2007 ; vol. 66, no. 4, pp. 458-463.
27. Canoruc C, Kale E, Turhanoglu A, (2009): Plasma
resistin and leptin levels in overweight and lean
patients with rheumatoid arthritis Turk J Med Sci
2009; 39 (3): 447-451.
28. Bokarewa M, Nagaev I, Dahlberg L, et al. Resistin,
an adipokine with potent proinflammatory properties.
J Immunol 2005; 174:5789-5795.
29. Kassem E, Mahmoud L, and Salah W, (2010): Study
of Resistin and YKL-40 in Rheumatoid Arthritis.
Journal of American Science 2010; 6(10): 31-35.
30. Elshishtawy H, ElDessouki Ibrahim S, Helmi A,
et al. Resistin in systemic lupus erythematosus,
Relation to lupus nephritis and premature atherosclerosis.
The Egyptian Rheumatologist 2012; 34, 137-146.
31. Migita K, Miyashita T, Maeda Y, et al. Toll-like
receptor expression in lupus peripheral blood mononuclear
cells. J Rheumatol 2007; 34:493.
32. Zhang Y, Juge-Aubry CE, Henrichot E, et al.
Adipose tissue: a regulator of inflammation. Best
Pract Res Clin Endocrinol Metab 2002; 19(4):547-66.
33. Yannakoulia M, Lee JH, Chan JL, et al. Circulating
resistin levels are not associated with obesity
or insulin resistance in humans and are not regulated
by fasting or leptin administration: cross-sectional
and interventional studies in normal, insulin-resistant,
and diabetic subjects. J Clin Endocrinol Metab 2003;
88(10):4848-56.
34. Gonzalez-Gay MA, Garcia-Unzueta MT , Gonzalez-Juanatey
C, et al. Anti-TNF-alpha therapy modulates resistin
in patients with rheumatoid arthritis, Clinical
and Experimental Rheumatology2008 ; 26, 2, 311-316.
35. Rho YH, Solus J, Sokka T. Adipocytokines Are
Associated with Radiographic Joint Damage in Rheumatoid
Arthritis. Arthritis Rheum 2009; July; 60(7): 1906-1914.
|
