|
Seroprevalence
of Herpes Simplex Virus Type 2 (HSV-2) in Pregnant
Women and its Relation to Some Blood Cells and
IL-2 in Kirkuk, Iraq
......................................................................................................................................................................
Abdulla Kamil Abdulla
Correspondence:
Abdulla Kamil Abdulla Supervisor: Dr. Israa H.
Saadoon/ Ph.D. Medical Microbiology
M.Sc. Medical Microbiology Medicine College/Tikrit
University
Erbil Health Directorate,
Iraq
Email: abdullakurdistan@yahoo.com
|
ABSTRACT
Background: The HSV-2, is a widespread
viral pathogen. It has been described as
an important etiological agent in uterus
and during the intrapartum period in pregnant
women.
Objectives: Estimate the prevalence
of HSV-2 antibodies among pregnant women
in Kirkuk city.
Patients and Methods: A cross sectional
study (M.Sc. Thesis) was conducted in Kirkuk
city and included 176 pregnant women, and
134 non-pregnant married women (control
group) who attended at Azadi Teaching Hospital
and Al Ta'akhi Health Care Center from the
20th of November 2012 to the 23rd of April
2013.
Results: The study revealed that
the 62.48 % of pregnant women were infected
with HSV-2. The highest rate of IgM antibodies
was found in 50% of pregnant women aged
18-23; this was also true for both IgM and
IgG antibodies together that were found
in 41.17% of women. The relation of seropositive
HSV-2 antibodies with the total white blood
cells (W.B.Cs) count showed a non-significant
result with the probability (P) value >0.05.
This was also true for the relation with
absolute lymphocyte count (ALC), while its
relation with absolute eosinophil count
(AEC) showed a significant result, P <0.05.
In regards to the relation of HSV-2 antibodies
with serum interleukin-2 (IL-2), the result
was non-significant. The relation with abortion
number was significant. There was significant
relation of abortion with gestational time
of pregnancy in seropositive pregnant women.
Conclusion: The seroprevalence of
HSV-2 was relatively high in pregnant women
in Kirkuk city. Primary and re-infection
of latency occurred at the highest rate
in age group 18-23 years old. Primary HSV-2
infection increases the AEC and IL-2 during
pregnancy. The highest rate of abortion
occurred during the first trimester of pregnancy
in women with HSV-2.
Key words: HSV-2, Iraq.
|
The HSV-2, is a widespread viral
pathogen. It has been described as an important
etiological agent in uterus and during intrapartum
period in pregnant women. The HSV-2 infection
has been found to be a sexually transmitted disease
affecting most commonly, individuals who are in
their adolescence or young adulthood[1,2].
The HSV-2 belongs to the Herpesviridae family.
The virion particle is spherical 150-200 nanometer
(nm) in diameter [3,4], with four structural elements;
an electron opaque core, a protein capsid, surrounding
the virus core comprising 162 capsomeres, an amorphous
tegument surrounding the capsid, and an outer
envelope with spikes on its surface. The core
is composed of linear dsDNA [5,6].
The primary route of acquisition of HSV-2 infections
is through genital sexual contact with an infected
partner who is shedding the virus symptomatically
or asymptomatically [7]. The HSV-2 infection is
more common in women than men[8].
Neonatal HSV-2 infection is acquired from the
mother during vaginal delivery [9]. The chances
of the baby becoming infected increase if there
is an outbreak at the time she delivers the infant[10].
The risk of transmission of HSV-2 during primary
infection in the third trimester of pregnancy
to the infant is estimated to be 30%-50%[11].
Intrauterine and postnatal transmissions are rare[12].
The HSV-2 virus is also associated with a higher
rate of miscarriages than normal[13]. Latency
of the virus is in sacral nerve ganglia[14]. The
HSV-2 can reactivate upon stress[15].
Direct detection of viral DNA by liquid or in
situ hybridization, and after, by the polymerase
chain reaction (PCR), are considerably more sensitive[16].
Enzyme-linked immunosorbent assay (ELISA) can
be used to detect immunoglobulin M and G (IgM
and IgG respectively) in serum[17].
Acyclovir is selectively effective against HSV-2.
Other drugs effective in treating HSV-2 infection
include famciclovir and topical Penciclovir[16].
Objectives: Estimate the prevalence of
HSV-2 antibodies among pregnant women in Kirkuk
city, and its relation to some blood cells and
IL-2.
Study Population:
A cross sectional study [M.Sc. thesis] conducted
in Kirkuk city included 176 pregnant women, and
134 non-pregnant married women (control group)
attending Azadi Teaching Hospital and Al Ta'akhi
Health Care Center from the 20th of November 2012
to the 23rd of April 2013 and aged (18-40) years
old. A blood sample of 7.5 ml was drawn from each
patient and separated into two parts; one part
5 ml was with no Ethylene Diamine Tetraacetic
Acid (EDTA) anticoagulant used for detection of
anti-HSV-2 IgM, IgG antibodies, and serum IL-2
using Enzyme Linked-Immunosorbant assay (ELISA)
technique, and the other part 2.5 ml was with
EDTA for detection of blood cells using specialized
fully automated hematological analyzer machine
(CELL-DYN RUBY).
Detection of anti-HSV-2 IgM and IgG antibodies
Enzyme Immunoassay for Detection of IgM antibodies
to HSV-2 in Human serum From BioCheck, Inc 323
Vintage Park Dr. Foster City, CA 94404.
Purified HSV-2 antigen is coated on the surface
of microwells. Diluted patient serum is added
to the wells, and the HSV-2 IgM-specific antibody,
if present, binds to the antigen. All unbound
materials are washed away. Horse radish peroxidase
(HRP-conjugate) is added, which binds to the antibody-antigen
complex. Excess HRP-Conjugate is washed off and
a solution of Tetramethyl Benzidine (TMB) reagent
is added. The enzyme conjugate catalytic reaction
is stopped at a specific time. The intensity of
the color generated is proportional to the amount
of HSV-2 IgM-specific antibody in the sample.
The results are read by a microwell reader compared
in a parallel manner with calibrator and control.
Samples: Serum (stored at -20 °C).
Enzyme Immunoassay for Detection of IgG Antibodies
to HSV-2 in Human Serum.
From BioCheck, Inc 323 Vintage Park Dr. Foster
City, CA 94404.
Purified HSV-2 antigen is coated on the surface
of microwells. Diluted patient serum is added
to the wells, and the HSV-2 IgG-specific antibody,
if present, binds to the antigen. All unbound
materials are washed away. HRP-conjugate is added,
which binds to the antibody-antigen complex. Excess
HRP-conjugate is washed off and a solution of
TMB reagent is added. The enzyme conjugate catalytic
reaction is stopped at a specific time. The intensity
of the color generated is proportional to the
amount of HSV-2 IgG-specific antibody in the sample.
The results are read by a microwell reader compared
in a parallel manner with calibrator and controls.
Samples: Serum (stored at -20 °C).
Detection of Human IL-2 in Human Serum
Enzyme Immunoassay for Detection of Human IL-2
in Human Serum.
From Biolegend Inc. Pacific Heights Blvd. San
diego, CA 92121
Human IL-2 EIA Kit is a sandwich enzyme immunoassay
(EIA) with a 96-well strip plate that is pre-coated
with a capture antibody. This kit is specifically
designed for the accurate quantization of human
IL-2 from cell culture supernatant, serum, plasma,
and other biological fluids. This kit is analytically
validated with ready-to-use reagents.
Samples: Serum (stored at -20 °C).
Detection of Blood Cells
The CELL-DYN Ruby uses flow cytometric techniques
to analyze the RBC/PLA, WBC, and nuclear optical
count (NOC) populations. Flow cytometry is a process
in which individual cells or other biological
particles in a single file produced by a fluid
stream are passed through a beam of light. A sensor
or sensors measure, by the loss or scattering
of light, the physical or chemical characteristics
of the cells or particles.
Samples: EDTA anticoagulant treated vein's
whole blood.
Statistical Analysis
Computerized statistical analysis was performed
using Mintab version 11 statistic program. Comparison
was carried out using; Chi-square (X2), and probability
(P value). The P value < 0.05 was considered
statistically significant, and for results where
its P value was less than 0.01 was considered
highly significant, while for those which its
P value was greater than 0.05 was considered non-significant
statistically.
Detection of anti-HSV-2 IgM
and IgG antibodies
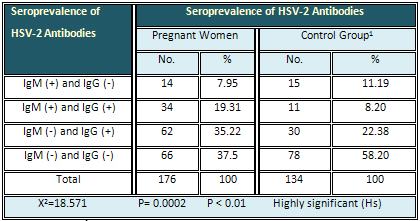
The current findings revealed that anti-HSV-2-IgM
was found in 7.95 % of pregnant women, anti-HSV-2-IgG
in 35.22 % and both IgM and IgG at the same time
in 19.31 %, while 37.3 % of them had neither IgM
nor IgG against HSV-2. Regarding the control group,
the rate of IgM, IgG, and both IgM and IgG (at
the same time) was 11.19%, 22.38 % and 8.2 % respectively.
However 58.2 % were negative for both IgM and
IgG. The result was highly significant (Table
1).
Table 1: Summary of the HSV-2 Antibodies Seroprevalence
in Pregnant Women and Control Group 1 (Non-Pregnant
Married Women)
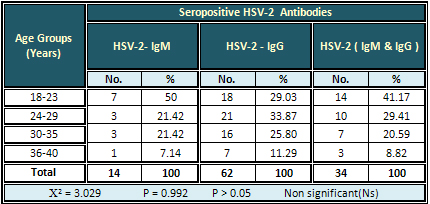
The highest rate (50 %) of HSV-2- IgM antibodies
was found in pregnant women aged 18-23 years,
while the highest rate (33.87 %) of HSV-2- IgG
antibodies was found in those aged 24-29 years.
Also the highest rate (41.17 %) of HSV-2- IgM
&IgG together was found in the age group 18-23
years. The result was non-significant (Table 2).
Table 2: Relation of Seropositive HSV-2 Antibodies
to Age Groups of pregnant Women
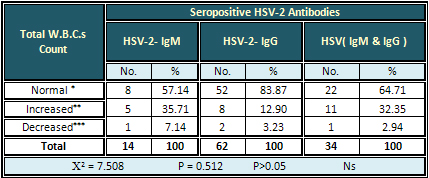
Detection of Blood Cells
Detection of total W.B.Cs
The highest rate (35.71%) of increased W.B.Cs
counts was seen with seropositive HSV-2-IgM antibodies.
The result was non-significant (Table 3).
Table 3: Relation of Seropositive HSV-2 Antibodies
with the Total W.B.C.s Counts
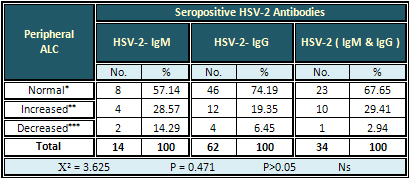
Detection of ALC
The highest rate (29.41 %) of increased to ALC
was found with HSV-2 (IgM & IgG) antibodies.
The result was non-significant (Table 4)
Table 4: Relation of Seropositive HSV-2 Antibodies
with Peripheral ALC
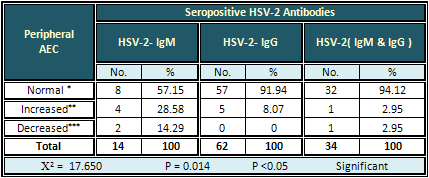
Detection of AEC
The highest rate (28.58%) of increased AEC was
found with HSV-2-IgM antibodies. The result was
significant (Table 5).
Table 5: Relation of Seropositive HSV-2 Antibodies
with Peripheral AEC
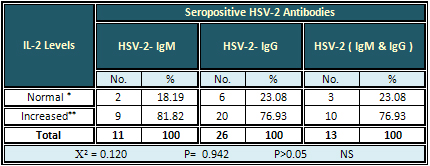
Detection of serum IL-2
The highest rate of increased IL-2 level was found
in all types of the HSV-2 antibodies and as following:
81.82 %, 76.93 %, and 76.93 % for HSV-2-IgM, HSV-2-IgG,
and HSV-2 (IgM & IgG) respectively. The result
was non-significant (Table 6).
Table 6: Relation of Seropositive HSV-2 Antibodies
with Serum IL-2 Levels
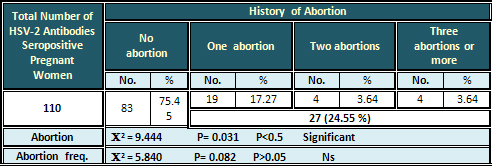
Relation of Anti-HSV-2 Antibodies
to History of Abortion, and Frequency of Abortion
The total rate of abortion was (24.55 %) out of
a total 110 seropositive pregnant women. The rate
of abortion number was 17.27 % for one abortion
and 3.64 % for each of two and three abortions
or more. The results were significant for abortion,
and non-significant for frequency of abortion
(Table 7).
Table 7: Relation of Seropositive HSV-2 Antibodies
in Pregnancy with History of Abortion and Frequency
of Abortion
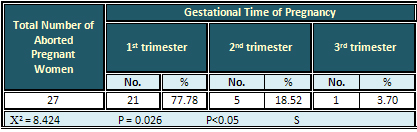
Relation of Abortion with
Gestational Time of Pregnancy in Pregnant Women
with Seropositive HSV-2 Antibodies
The highest rate (77.78 %) of abortion was found
in the 1st trimester, while the lowest rate was
found in the 3rd trimester. The result was significant
(Table 8).
Table 8: Relation of Abortions with Gestational
Time in Pregnant Women with Seropositive HSV-2
Antibodies
The HSV-2 is the leading cause
of genital ulcer disease worldwide. The virus
can be transmitted to neonates[18]. Maternal-fetal
transmission of HSV-2, which is frequently asymptomatic,
can cause severe and permanent neurological damage
to the neonate[19]. The prenatal form of the infection
in newborns can be observed when, a neonate passes
through the infected birth canal. Contamination
of the neonate with this condition may cause meningitis
with a serious complication[20]. Furthermore and
according to our information, no such study of
similarity has been published regarding pregnant
women with HSV-2 infection in Iraq.
In the present study, the HSV-2 infection was
relatively common among pregnant women. ELISA
method was used as a serological method for detection
of seropositive HSV-2 antibodies, and then the
results were classified according to seropositive
HSV-2 antibodies type to: HSV-2-IgM represented
the acute state of (primary) infection, HSV-2-IgG
represented the past (chronic) infection, and
both HSV-2-IgM & HSV-2-IgG at the same time
represented the re-infection or reactivation of
latent infection[21].
The rate of seropositive anti-HSV-2-IgM antibodies
obtained by the current study was similar to that
obtained from other Iraqi cities like Baghdad
(8.1 %) and Waset province (7.7 %), but slightly
lower than that recorded in Mosul (10 %)[22,23,24].
In Turkey, it was 8.2% which is close to our findings
too, but in another study in Turkey, it was 11.2
% which is slightly higher than that recorded
by the current findings[25]. These variations
in results may be attributed to the fact that
different ELISA kits used in the other studies
from different companies may be with different
reagents qualities and properties. Other factors
which may also be attributed to the differences
are steps, and techniques used by the investigators.
While our finding was higher than that recorded
in Saudi Arabia 0.5 %[26], this may be due to
the lack of a nationwide screening program in
our country to control the infection, which maybe
Saudi Arabia has. Results obtained by the current
study were largely lower than that reported in
northern India (33.5 %), [27], in which most people
from this area are known to have a very low living
standard, this high rate may be an indication
that the HSV-2 infection may be endemic in this
area.
Regarding the anti-HSV-2-IgG antibodies rates;
the present study revealed that, the rate of anti-HSV-2-IgG
antibodies was 35.22 % of the pregnant women.
This result was similar to those reported in Waset
province (31.3 %), Tanzania (33 %) and Sweden
(34 %)[23][28,29]. While the rate was lower than
that recorded in Turkey (63.1 %), Iran (43.75
%), and Uganda (86 %)[25][29,30]. This may be
related to different cultural factors and different
socioeconomic factors too. In addition it may
be associated with co-infection of HSV-2 with
other viruses infections that enhance the transmission
and increase the prevalence of HSV-2, especially
HIV which has the same route of transmission and
is present at high rates in these areas and may
be endemic. The current findings were higher than
that recorded In Japan (7 %), Italy (7.6 %), USA
(22 %), and Germany (18 %)[28][31,32]. This may
be attributed to the fact that these countries
are considered as developed countries, and may
have good nationwide surveillance programs to
control the infection. The HSV-2 infection has
a high prevalence rate in pregnant women in developing
countries, especially those with a high rate of
HIV prevalence[33].
The rate of both anti-HSV-2 (IgM & IgG at
the same time) antibodies in pregnant women in
the present study was 19.31 %. This was higher
than that recorded in India (2.9 %)[34,35]. This
may be due to the fact that in India, a safety
program may have been developed for pregnant women
to protect them from HSV-2 infection by following
some special criteria like examining those mothers
who got primary infection in the past and were
at great risk of reactivation during pregnancy.
Primary infection with HSV-2 acquired by women
during pregnancy accounts for a half of the morbidity
and mortality from HSV-2 among neonates, and the
other half results from reactivation of old infection.
(24) Since there are physiological changes during
pregnancy that might affect the hormone levels;
hormones like progesterone for instance may increase
the susceptibility and decrease the immune response
to genital herpes infection[36].
In the current study the highest rate (50 %) of
seropositive anti-HSV-2-IgM antibodies was found
in pregnant women aged 18-23 years. This was also
true for the seropositive anti-HSV-2 (IgM &IgG)
antibodies which were 41.17 % in pregnant women
aged 18-23 years (as shown in Table 2). Age is
one of the determinant factors associated with
the prevalence of HSV-2[37]. Ashley, et al, [38],
said that the acquisition of primary infection
of HSV-2 increases in earlier ages, less than
the third decade of life. This also agrees with
Sen, et al [27]. The reason may be due to the
fact that most pregnancies occur at this age.
In addition to that, this age group may have more
contact with infected persons.
Data obtained by the current work revealed that
the re-infection and reactivation had also occurred
at a highest rate in age group 18-23 years. This
may be associated with some factors like stress,
hormonal changes, especially most of these women
have married recently, so once they got married
and pregnant a lot of physiological changes may
be happening in their bodies which make them more
vulnerable to the infection. The highest rate
of anti-HSV-2-IgG antibodies was 33.87 %, which
was lower than that recorded in Colombia (64.3
%), and in Thailand (36.8 %)[39]. This may be
due to socio-demographic reasons, and most women
in these areas might have been infected with the
virus at younger ages. Although the age was the
determinant factor influencing HSV-2 seroprevalence,
the results were non-significant (P > 0.05),
in correlation with age groups which was disagreed
with by Smith, et al [40]. This is may be due
to low differences in demographic distribution
of the virus in our society compared to the other
countries.
Regarding the total W.B.Cs count; the current
study agrees with Lakhan, et al, [41] who found
normal W.B.Cs count in HSV-2 positive patients.
On the other hand, Navaneethan, et al, [42] recorded
a higher rate of decreased W.B.Cs in seropositive
HSV-2 pregnant women. These differences may be
due to the fact that pregnant women are particularly
susceptible as immunological changes during pregnancy
suppress T-cell mediated immunity promoting disseminated
infection like HSV-2 hepatitis.
The current study showed that the highest rate
of normal ALC was found with all types of the
anti-HSV-2 antibodies, while the highest rate
of increased ALC was found with seropositive anti-HSV-2
(IgM & IgG together) antibodies. The HSV-2
is considered one of the infectious agents that
lead to lymphocytosis and increase in the peripheral
ALC[43,44]. Although lymphocytes increased during
HSV-2 infections in pregnant women, the result
was non-significant (P > 0.05) (as shown in
Table 4). This may be due to the differences in
kinetic responses of the lymphocyte cells in these
pregnant women. In addition, these pregnant women
may have other viral infections or hematological
conditions that affect the response of the lymphocytes.
These findings agree with Koelle, et al, [45]
who recorded non-significant (P > 0.05) results
regarding the relation of HSV-2 infection with
lymphocytes count.
The present study showed that the highest rate
of normal AEC was found with all types of the
anti-HSV-2 antibodies, while the highest rate
of increased AEC was found in pregnant women who
were seropositive for anti-HSV-2-IgM antibodies.
The result was significant (P < 0.05) (as shown
in Table 5). The HSV-2 infection is associated
with eosinophilia[46]. This may be attributed
to the fact that HSV-2 causes severe itching when
infecting immune-compromised individuals such
as pregnant women leading to increase in the eosinophils
count. This finding agrees with Tarkkanen, et
al[47], who recorded high eosinophil count in
relation with seropositive anti-HSV-2 antibodies.
Eosinophilia may be associated with HSV-2 infections
because of its effects on the skin causing rashes,
on the eyes, on the genitalia and so on[48].
The IL-2 is a cytokine secreted by Th1 cells[49].
Although the current study showed that the highest
rate of increased IL-2 was found with all types
of the anti-HSV-2 antibodies, there were non-significant
(P > 0.05) differences in the results in regards
to the relation of IL-2 levels with the seropositive
anti-HSV-2 antibodies in the pregnant women (as
shown in Table 6). This agrees with Rushbrook,
et al[50], who said that there was no relation
between IL-2 levels and HSV-2 severity. In other
studies using whole HSV antigen, adults who had
a better IFN- response during genital HSV infection
had a longer interval to recurrence, and recurrences
have been associated with decreased IL-2 production
induced by HSV antigen[51]. These differences
in the results may be due to the differences in
the ability of HSV-2 to switch the Th2 cells to
Th1, the latter which are responsible for secreting
of IL-2, in pregnant women. Besides, these women
may have had other infections that led to the
changes in IL-2 secretion, and as a result these
infections led to the differences in the results
that have been noted above.
The acquisition of genital herpes during pregnancy
has been associated with spontaneous abortion,
prematurity, and congenital and neonatal herpes[52].
The present study showed that the highest rate
of seropositive anti-HSV-2 antibodies was found
in pregnant women who have had no history of abortion,
followed by those who had a history of one abortion
and showed significant (P < 0.05) results in
regards to the history of abortion with seropositive
anti-HSV-2 antibodies (as shown in Table 7). This
agrees with Kim, et al [53], who recorded significant
difference in regards to HSV-2 infections with
a history of abortion.
Regarding the frequency and recurrent abortion
due to HSV-2 infection in pregnant women; the
current study showed non-significant (P > 0.05)
difference in number of abortions among single,
twice, and three times or more of abortion frequency
( as shown in Table 7). This agrees with Jasim,
et al[23] who observed a non-significant relation
in regards to abortion frequency and seropositive
anti-HSV-2 antibodies. These findings point to
that acute infection or reactivation of latent
infection of HSV-2 that may occur as a result
of immune suppression or certain physiological
changes in the body during pregnancy.
Furthermore to have a safe pregnancy there has
to be a switch from Th1 to Th2 and not the other
way around, and this is due to the fact that Th1
cytokines are considered to be detrimental to
pregnancy, via direct embryo toxic activity, or
via damage to the placental trophoblast, or possibly
by activating cells that are deleterious to the
conceptus, whereas Th-2 cytokines may directly
or indirectly contribute to the success of pregnancy
by down regulating potential Th-1 reactivity[54].
The present study showed that the highest rate
(77.78 %) of abortion was found in the first trimester
of pregnancy, and the result was significant (P
< 0.05) (as shown in Table 8). This agrees
with Borhani, et al [55] who said; the danger
of intrauterine HSV transmission is highest during
the first trimester of gestation and it can lead
to abortion, stillbirth and congenital anomalies.
The differences in results may be due to some
maternal infections, such as CMV, especially during
the early gestation, which can result in fetal
loss or malformations because the ability of the
fetus to resist infectious organisms is limited
and the fetal immune system is unable to prevent
the dissemination of infectious organisms to various
tissues. The fetus and/or neonate are infected
predominantly by viral and also by bacterial and
protozoal pathogens. Infections with various pathogens
cause miscarriage or may lead to congenital anomalies
in the fetus while others are associated with
neonatal infectious morbidity[56].
The seroprevalence of HSV-2 was
relatively high in pregnant women in Kirkuk city.
Primary and re-infection of latency occurred at
highest rate in age group 18-23 years old. Primary
HSV-2 infection increases the AEC and IL-2 during
pregnancy. The highest rate of abortion occurred
during the first trimester of pregnancy in women
with HSV-2.
1- Kelika A, Konda B, Jeffrey D, et al. The Epidemiology
of herpes simplex virus type 2 infection in low-income
urban populations in coastal Peru. Sex Trans Dis
2005; 32: 534-541.
2- Mahy's Virology dictionary. 3rd ed. Atlanta,
Georgia: Acadmic press 2001: 186-187.
3- Drannik A. Characterization of antiviral properties
of trappin-2 and elafin against HIV-1 and HSV-2
in the female genital mucosa [Ph.D. thesis]. Hamilton,
Ontario: University of McMaster 2012.
4- Orosz L. The effect of vesicular stomatitis virus
and herpes simplex virus infection on the expression
patterns of p63 and Bax in different epithelial
cell lines [Ph.D. thesis]. Szeged, Hungary: University
of Szseged, Faculty of Medicine 2010.
5- Zuckerman A, Banatvala J, Schoub B, Griffiths
P, Mortimer P. Principles and practice of clinical
Virology. 6th ed. Oxford, UK: John Wiley and Sons
Ltd; 2009: 95-96.
6- Zuckerman A, Banatvala
J, Schoub B, Griffiths P, Pattison J. Principles
and practice of clinical Virology. 5th ed. Oxford,
UK: John Wiley and Sons Ltd; 2004: 27-29.
7- Kimberlin D, Rouse D. Genital herpes. N Eng J
Med 2004 350:1970-1971.
8- American AIDS education and training center.
Herpes simplex cold sores and genital herpes. New
Mexico, USA: National Library of Medicine; 2012.
9- Tidy C. Congenital, perinatal and neonatal infections.
London, UK: Egton Medical Information Systems Limited;
2013: 3-7.
10- Counseling messages for herpes simplex type
2 genital herpes. Centers for Disease Control and
Prevention; 2002: 3-8.
11- Herpes simplex virus type 2 programmatic and
research priorities in developing countries. London:
World Health Organization and the Joint United Nations
Programme on HIV/AIDS; 2001: 8-19.
12- LiHsu W. Herpes simplex virus type 1 infection
and ND10 characteristics in cultured fibroblast
and neuronal-like cells [Ph.D. thesis]. Scotland,
UK: University of Glasgow 2002.
13- Prescott L, Harley J, Klein D. Microbiology.
5th ed. Boston, U.S.A: The McGraw-Hill Companies;
2002: 886-887.
14- Todd P, Yumi I, Li Y, Vallas V, Krause P. Herpes
simplex virus type 2 establishes latent infection
in a different population of ganglionic neurons
than HSV-1, role of latency associated transcripts.
J Virol 2007; 81: 1872-1878.
15- Goldman E, Green L. Practical Handbook of Microbiology.
2nd ed. London, UK: Chemical Rubber Company Press;
2009: 807-808.
16- Harvery R, Champe P, Fisher B. Lippincott's
illustrated reviews: Microbiology. 2nd ed. Baltimore,
Md: Lippincott Williams and Wikins; 2007: 261-262.
17- Nick S, Metzger B, Muller S, Falke D. Virus
specific IgM and IgG antibody production by B cells
during herpes simplex virus type 2 induced immunosuppression
as analysed by an immunospot assay. J Gen Virol
1987; 68: 1951-1959.
18- Munjoma M, Kurewa E. The prevalence, incidence
and risk factors of herpes simplex virus type 2
infection among pregnant Zimbabwean women followed
up nine months after childbirth. Wom heal 2010;
10: 2-4.
19- Leylan B, Kennedy M, Wimberly Y, Levine B, Cherpes
T. Serologic detection of herpes simplex virus type
2 antibodies among pregnant women using a point-of-care
test from Focus diagnostics. J Clin Virol 2009;
44: 125-128.
20- Ziyaeyan M, Japoni A, Roostaee M, Slehi S. Soleimanjahi
H. A serological survey of herpes simplex virus
type 1 and 2 immunity in pregnant women at labor
stage in Tehran, Ira Pak J Bio Sci 2007; 10: 148-151.
21- Butel S, Morse A. Herpes viruses. In: Jawetz,
Melnick and Adelberg's (eds.). Medical Microbiology.
23rd ed. McGraw-Hill company 2004: 440-446.
22- Abdul Mohymen N, Hussein A, Hassan F. Association
between TORCH agents and recurrent spontaneous abortion.
Iraqi J Med Sci 2009; 7 40-46.
23- Jasim M, Majeed H, Ali A. Performance of serological
diagnosis of TORCH agents in aborted versus non
aborted women of Waset province in Iraq. Tik Med
J 2011; 17: 141-147.
24- Al-Taie A. Serological study for TORCH infections
in women with high delivery risk factors in Mosul.
Tik J Pur Sci 2010; 15: 193-196.
25- Duran N, Yarkin F, Evruke C, Koksal F. Asymptomatic
herpes simplex virus type 2 infection among pregnant
women in Turkey. Ind J Med Res 2004; 120: 106-110.
26- Alzahrani A, Obeid O, ALmulhim A, et al. Analysis
of herpes simplex 1 and 2 IgG and IgM antibodies
in pregnant women and their neonates. J Fam Com
Med 2007; 14: 3-7.
27- Sen M, Shukla B, Banerjee T. Prevalence of serum
antibodies to TORCH infection in and around Varanasi,
Northern India. J Clin Diag Res 2012; 6: 1483-1485.
28- Kasubi M, Nilsen A, Marsden H, Bergstrom T,
Langeland N, Haarr L. Prevalence of antibodies against
herpes simplex virus type 1 and 2 in children and
young people in an urban region in Tanzania. J Clin
Microbiol 2006; 44: 2801-2807.
29- Msuya S, Mbizvo E, Hussain A, et al. Seroprevalence
and correlates of herpes simplex virus type 2 among
urban Tanzanian women. Sex Trans Dis 2003; 30: 588-592.
30- Shahraki A, Moghim S, Akbari P. A survey on
herpes simplex virus type 2 antibody among pregnant
women in Isfahan, Iran. J Res Med Sci 2010; 15:
243-244.
31- Anzivino E, Fioriti D, Mischitelli M, et al.
Herpes simplex virus infection in pregnancy and
in neonate: status of art of epidemiology, diagnosis,
therapy and prevention. J Virol 2009; 6: 1-4.
32- Sauerbrei A, Schmitt S, Scheper T, et al. Seroprevalence
of herpes simplex virus type 1 and 2 in Thuringia,
Germany, 1999 to 2006. Euro Surveill J 2011; 16:
6-7.
33- Nakubulwa S, Mirembe F, Kaye D, Kaddu-Mulindwa
D. Association between HSV-2 and HIV serostatus
in pregnant women of known HIV serostatus attending
Mulago hospital antenatal clinic, Kampala, Uganda.
J Infet Dev Ctries 2009; 3: 803-806.
34- Mahalakshmi B, Therese K, Devipriya U, Pushpalatha
V, Margrita S, Madhavan H. Infectious aetiology
of congenital cataract based TORCHES screening in
a tertiary eye hospital in Chennai, Tamil Nadu,
and India. Ind J Med Res 2010; 131: 559-564.
35- Kropp R, Wong T, Cormier L, et al. Neonatal
herpes simplex virus infections in Canada, results
of a 3 years national prospective study. Ped J 2006;
117: 1955-1956.
36- Kaushic C, Ashkar A, Reid L, Rosenthal K. Progesterone
increases susceptibility and decreases immune responses
to genital herpes infection. J Virol 2003; 77: 4558-4565.
37- Margan M, Chicin G, Moldovan R. Clinical and
epidemiological considerations on herpes simplex
genital infections. J Hyg Pub Heal 2009; 59: 7-8.
38- Ashley R, Wald A. Genital herpes: Review of
the epidemic and potential use of type-specific
serology. Clin Microbiol Rev 1999; 12: 1-8.
39- Patnaik P, Herrero R, Morrow R, et al. Type-specific
seroprevalence of herpes simplex virus type 2 and
associated risk factors in middle-aged women from
6 countries: The IARC multicentric study. Sex Trans
Dis 2007; 34: 2-3.
40- Smith J, Rosinska M, Trzcinska A, Pimenta J,
Litwinska B, Siennicka J. Type specific seroprevalence
of HSV-1 and HSV-2 in four geographical regions
of Poland. Sex Trans Inf 2006; 82: 159-163.
41- Lakhan S, Harle L. Fatal fulminant herpes simplex
hepatitis secondary to tongue piercing in an immunocompetent
adult: a case report. J Med Cas Rep 2008; 2: 356.
42- Navaneethan U, Lancaster E, Venkatesh P, Wang
J, Neff G. Herpes simplex virus hepatitis- it's
high time we consider empiric treatment. J GIT Liv
Dis 2011; 20: 93-96.
43- Hoffbrand A, Moss P, Pettit J. Essential Hematology.
5th ed. Massachusetts, USA: Blackwell; 2006: 117-119.
44- America lab tests. Complete blood count. Washington,
USA: American association for clinical chemistry;
2012: 2-3.
45- Koelle D, Posavad C, Barnum G, Johnson M, Frank
J, Corey L. Clearance of HSV-2 from recurrent genital
lesions correlates with infiltration of HSV-specific
cytotoxic T lymphocyte. J Clin Inves 1998; 101:
1500-1508.
46- England Affinity and society. Assessment of
eosinophilia; London, UK: Brit Med J publishing
group limited; 2011.
47- Tarkkanen A, Laatikainen L. Late ocular manifestations
in neonatal herpes simplex virus infection. Brit
J Ophth1977; 61: 608-616.
48- Pettay O, Leinikki P, Donner M, Lapinleimu K.
Herpes simplex virus infection in the newborn. Arch
Dis Child 1972; 47: 97.
49- Toliver-Kinsky T, Lin C, Herndon D, Sherwood
E. Stimulation of hematopoiesis by the Fms-like
tyrosine kinase 3 ligand restores bacterial induction
of Th1 cytokines in thermally injured mice. Inf
Immun 2003; 71: 3058- 3067.
50-Rushbrook S, Ward S, Unitt E, et al. Regulatory
T cells suppress in vitro proliferation of virus
specific CD8 T cells during persistent Hepatitis
C Virus infection. J Virol 2005; 79: 7852-7859.
51- Carmack M, Yasukawa L, Chang S. T cell recognition
and cytokine production elicited by common and type-specific
glycoproteins of herpes simplex virus type 1 and
2. J Inf Dis 1996; 174: 899-906.
52- Apurba S, Sandhya B, Senthamarai S, et al. Serological
evaluation of herpes simplex virus type 1 and 2
infections in pregnant women with bad obstetric
history in a tertiary care hospital, Kanchipuram.
Inter J Adv Res 2013; 1: 123-128.
53- Kim D, Chang H, Hwang K. Herpes simplex virus
type 2 infection rate and necessity of screening
during pregnancy: A clinical and seroepidemiologic
study. Yons Med J 2012; 53: 401-407.
54- Ahmed D. Effects of Interleukin-2 (IL-2) and
Interleukin-6 (IL-6) in recurrent spontaneous abortion.
J Virol 2008; 17: 74-76.
55- Borhani M, Hosseini S, Chamani-Tabrizi L, et
al. PCR detection of herpes simplex virus in human
placenta and aborted material with spontaneous abortion.
Iran J Clin Inf Dis 2011; 6: 18-20.
56- Haider M, Rizvi M, Khan N, Malik A. Serological
study of herpes virus infection in female patients
with bad obstetric history. Bio Med 2011; 3: 284-290.
|
