|
Predictive value
of pain intensity in the clinical severity of
painful crises in children and adolescents with
sickle cell diseases
......................................................................................................................................................................
Can Acipayam (1)
Bayram Ali Dorum (2)
Gül Ilhan (3)
Ali Ersoy (2)
Gönül Oktay (4)
Mehmet Rami Helvaci (5)
(1) Department of Pediatric
Hematology and Oncology,
Medical Faculty of Mustafa Kemal University, Hatay,
Turkey.
(2) Department of Pediatric, Medical Faculty of
Mustafa Kemal University, Hatay, Turkey.
(3) Department of Hematology, Hatay Antakya State
Hospital, Hatay, Turkey.
(4) Hemoglobinopathy Center, Hatay Antakya State
Hospital, Hatay, Turkey.
(5) Department of Internal Medicine, Medical Faculty
of Mustafa Kemal University, Hatay, Turkey.
Correspondence:
Can Acipayam, MD
Department of Pediatric Hematology and Oncology
Mustafa Kemal University, Tayfur Ata Sokmen Medical
School,
Serinyol, Hatay 31000, Turkey.
Phone Number: +90 326 229 10 00
Fax Number: +90 326 245 56 54
Email: cacipayam@hotmail.com
|
ABSTRACT
Objectives: Painful crisis is a significant
problem for patients with sickle cell diseases
(SCD). We tried to understand whether or
not there is an association between severity
of pain and complication rate in hospitalized
children and adolescents with SCD in the
present study.
Methods: All hospitalized SCD patients
with painful crisis between September 2012
and September 2013 were included into the
study. The intensity of pain was assessed
at the first visit. Pain scores were obtained
using the Faces Pain scale and Verbal Descriptor
Scale. Severity of pain was divided into
three groups as mild, moderate, and severe
according to the scales.
Results: Seventy-nine patients under
the age of 18 years-old with SCD and 146
episodes of painful crisis were evaluated.
Forty-five (57%) patients were women and
mean age was 11.5 years. The white blood
cell counts, aspartate aminotransferase
and C-reactive protein (CRP) were significantly
higher while erythrocytes, hemoglobin, hematocrit
and albumin levels were significantly lower
in the severe pain episodes group (p<0.05
for all). The number of patients transfused
was significantly high in the severe pain
episodes group than the other two groups
(p=0.006, p=0.001). Most of severe pain
episodes group had complicated vaso-occlusive
crisis (acute chest syndrome 41.6 %, Hepatic
sequestration crisis 6.7%), (p<0.05).
Conclusion: There may be an direct
relationship between prevalence of complicated
vaso-occlusive crisis and pain intensity
of SCD. Patients with sickle cell anemia
should be classified according to their
pain scores during hospitalization, and
patients with high pain scores should be
closely monitored for complications.
Key words: Sickle cell diseases,
Vaso-occlusive crisis, Severity of pain,
Pain score
|
Sickle cell disease (SCD) is
inherited as an autosomal recessive disorder.
It is now well established that SCD results from
a single change of one amino acid, valine, instead
of glutamic acid at the sixth position of the
hemoglobin beta chain. The prevalence in Turkey
as a whole is 0.3-0.6%, although this rises to
3%-44% in some parts of the Çukurova region.(1)
SCD is characterized by chronic hemolytic anemia,
dactylitis, and acute episodic clinical events
known as "crises." Vaso-occlusive (painful)
crises (VOC) are the most common and start in
infancy and early childhood. Other crises are
acute chest syndrome, central nervous system crisis,
sequestration crisis and aplastic crisis. The
factors that precipitate or modulate the occurrence
of sickle cell crisis are not fully understood,
but infections, hypoxia, dehydration, acidosis,
stress and cold are believed to play some role.
Frequent episodes of crisis, infections and organ
damage reduce the quality of life of patients
with SCD. A high rate of VOC is an index of clinical
severity that correlates with early death.(1,2)
VOC is also the most prevalent complication of
SCD. Pain is the insignia of SCD. Acute VOC is
a common medical emergency in patients with SCD,
necessitating hospitalization. Tissue damage due
to vaso-occlusion releases numerous inflammatory
mediators that initiate the transmission of painful
stimuli and the perception of pain. Sickle cell
vaso-occlusion, which may involve both the micro-
and macrovasculature, is the most important pathophysiological
event in SCD and explains most of its clinical
manifestation.(3)
The decision to admit a patient with SCD requires
multi-modal evaluation of severity of anemia,
presence of infection, priapism, acute chest syndrome,
acute stroke or another life-threatening complication.(4)
The pain severity ratings Visual Analog Scale
(VAS), Numeric Rating Scale (NRS), Verbal Descriptor
Scale (VDS) and Faces Pain Scale (FPS) are used.
The Verbal Descriptor Scale (VDS) is based on
the patient selecting the most appropriate word
to describe his/her condition. Pain is classified
as mild, moderate or severe on a simple pain scale.(5-7)
VAS and NRS are used with patients with SCD.(8)
Jones et al.(6) converted pain-intensity scores
associated with the Bieri FPS, NRS and VDS into
four levels (none, mild, moderate, and severe)
to analyze the effectiveness of a pain intervention.
Painful crisis is a significant problem for children
with SCD, and there has been little progress to
date in its management. There are insufficient
studies concerning pain severity scores and prevalence
of VOC complications in patients with severely
painful crises, and also the management of these
patients. The purpose of this study was to estimate
the effect of initial pain severity ratings on
progress, complications and management of hospitalized
pediatric patients with SCD.
This study comprised a retrospective
chart review of a single-center series of 79 patients
with SCD. The sample studied included all pediatric
patients hospitalized at Antakya State Hospital,
Turkey, between September 2012 and September 2013,
with SCD with painful crisis. Data collected included
demographic characteristics, clinical, hematological
and biochemical data, and Verbal Descriptor Scale
and Face Pain Scale scores. Data were obtained
from patients' medical charts. The following clinical
variables were recorded: age, gender, length of
hospital stay (days), duration of pain (days),
fever (axillary temperature equal to or greater
than 38.0 oC), transfusion, exchange transfusion,
type of pain crisis, factors triggering the painful
crisis and intensity of pain.
Baseline values for hematological parameters,
and liver and renal functions were recorded. Blood
samples were collected in EDTA containing tubes
for measurement of leukocyte (WBC), erythrocyte
(RBC), hemoglobin (HGB), hematocrit (HCT) and
platelet (PLT) levels. A complete blood count
(CBC) was carried out on an automated hematology
analyzer (Sysmex XT- 2000i, USA). Biochemical
parameters (glucose, blood urea nitrogen (BUN),
creatinine, aspartate aminotransferase (AST),
alanine aminotransferase (ALT), total bilirubin,
direct bilirubin, protein and albumin) were assessed
in blood samples. All biochemical investigation
was performed on a Modular Analytics P800 analyzer
(Roche Diagnostics, Indianapolis, IN) using spectrophotometric
methods. Concentrations of serum C-reactive protein
(CRP) in 146 samples were measured by nephelometry
using a BN II Nephelometer (Siemens). Serum CRP
values were considered normal between 0 and 5
mg/dl.
Intensity of pain was assessed at the first visit,
before any analgesia was administered. Pain scores
were obtained using the Faces Pain Scale (FPS)
and Verbal Descriptor Scale (VDS). Pain severity
based on the Verbal Descriptor Scale was based
on the patient selecting the most appropriate
word for his/her condition. Pain was classified
as mild, moderate or severe on the basis of FACES
Pain Scale and VSD pain scores (VDS: no pain;
slight and mild pain = mild pain; moderate pain;
severe pain, very severe pain, and most intense
pain possible = severe pain. Bieri FPS: face 1
= no pain, faces 2-3 = mild pain, faces 4-5 =
moderate pain, faces 6 -7 = severe pain).(5-7)
For statistical analysis, patients were divided
into three groups: one group with mild pain, one
group with moderate pain and one group with severe
pain. Patients were compared according to severity
of pain in terms of demographic, clinical, hematological
and biochemical parameters.
Statistical analysis
Statistical analyses were performed on SPSS software,
version 15. Data are expressed as arithmetic mean
± standard deviation (SD) for quantitative
data and as percentages (%) for qualitative data.
Values are presented as mean (minimum-maximum).
The Mann-Whitney U test or Chi-square test was
used for comparisons between groups, as appropriate.
Categorical variables were assessed using the
Pearson's chi-square test. A p value of <0.05
was considered significant.
One hundred and forty-six episodes
of painful crisis in 79 patients were evaluated.
Forty-five (57%) patients were girls and 34 (43%)
boys. Mean age was 11.5 years. Mean number of
painful crises per year per patient was 1.8. Patients'
demographic characteristics are summarized in
Table 1. Painful episodes most often involved
vaso-occlusive crisis (n=91, 62.3%), followed
by acute chest syndrome (n=35, 24%), splenic sequestration
crisis (n=15, 10.3%) and hepatic sequestration
crisis (n=4, 2.7%). Only one patient experienced
central nervous system crisis (0.7%). Mean hospital
length of stay for the 146 painful crisis episodes
was 5.5±3.5 days, and mean duration of
pain was 4.2±1.9 days.
The mild pain group experienced 15 (11%) episodes,
the moderate pain episodes group 71 (48%) and
the severe pain group 60 (41%). Age and gender
distribution were similar between the groups.
Mean length of hospital stay was significantly
higher in the severe pain group than in the mild
and moderate pain groups (p=0.001, p=0.001). Mean
duration of pain was significantly longer in the
severe pain group than in the other two groups
(p=0.003, p=0.028). Fever was highest in the severe
pain group (p<0.05). The number of patients
transfused was also significantly higher in the
severe pain group than in the other two groups
(p=0.006, p=0.001). Eight patients were treated
with exchange transfusion, six of whom were in
the severe pain group (p>0.05). Most of the
moderate pain group experienced VOC (77%), while
most of the severe pain group had acute chest
syndrome (41.6%) (p<0.05). Hepatic sequestration
crisis occurred in four patients (6.7%) in the
severe pain group. There were also significant
differences between this group and the moderate
pain group (p=0.027). Stress was the most common
trigger of painful episodes in the mild pain group,
and infection in the severe pain group (p=0.001,
0.002). Details of comparative values are given
in Table 2.
Hematological and biochemical values were similar
in the mild and moderate pain groups. Sickle cell
anemia patients with severe pain episodes had
significantly higher WBC and CRP levels compared
with the other two groups (p=0.014, p=0.003, p=0.025,
p=0.004, respectively). Erythrocyte count was
significantly lower in the severe pain group than
in the mild pain group (p=0.008). Sickle cell
anemia patients with severe pain episodes had
significantly lower HGB and HCT levels compared
with the moderate pain group (p=0.002, p= 0.002).
Aspartate aminotransferase levels were significantly
higher in the severe pain group (p= 0.022) and
albumin levels significantly lower (p=0.001) than
in the moderate pain group. Normal range creatinine
was higher in the severe pain group compared with
the moderate pain group (p=0.04). The hematological
and biochemical values of the groups are presented
in Table 3.
Table 1. Patients' demographic characteristics.
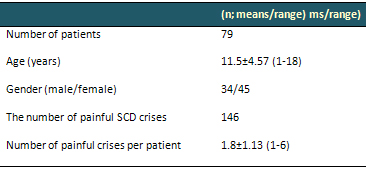
Data are arithmetical means ± SD. SCD;
Sickle cell disease
Table 2: Patients' clinical characteristics
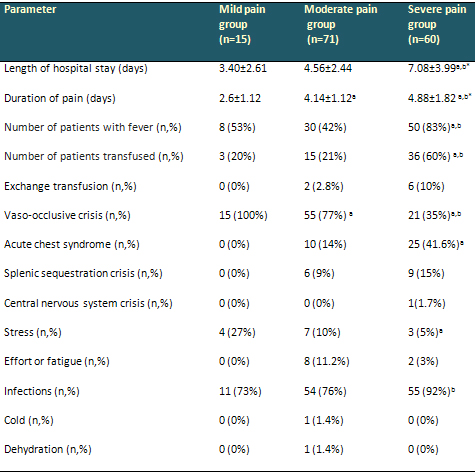
Data are arithmetical means ± SD (standard
deviation). a Statistically significant at p <
0.05 compared to the mild pain group. b Statistically
significant at p < 0.05 compared to the moderate
pain group. *p-values were calculated by Mann-Whitney
U test. Other p-values were calculated by Pearson's
chi-square test.
Table 3: Hematological and biochemical parameters
of patients
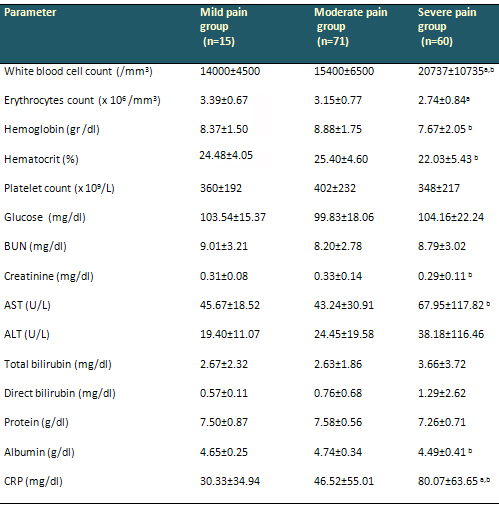
Data are arithmetical means ± SD (standard
deviation). a Statistically significant at p <
0.05 compared to the mild pain group. b Statistically
significant at p < 0.05 compared to the moderate
pain group. BUN; blood urea nitrogen, AST; Aspartate
aminotransferase, ALT; Alanine aminotransferase
, CRP; C-reactive protein. p-values were calculated
by Mann-Whitney U test.
Patients with SCD with pain crises
were hospitalized in this study. Patients were
divided into three groups on the basis of pain
assessment scales. Length of hospitalization and
duration of pain, WBC, AST and CRP were significantly
higher in the SCD group with severe painful episodes,
while RBC, hemoglobin, hematocrit and albumin
values were significantly lower. More complicated
VOC was also observed in this group.
Patients with SCD suffer from acute, painful vaso-occlusion
crises, infections and life-threatening acute
chest syndrome. Vaso-occlusive crises are the
most common causes of acute morbidity and medical
emergency in sickle cell anemia patients requiring
hospitalization.(9-11) Lionnet F et al.(12) reported
prevalences of hospitalized painful VOC, acute
chest syndrome and priapism of 36%, 20% and 20%,
respectively. In our study frequencies of hospitalized
painful VOC, acute chest syndrome, splenic sequestration
crisis, hepatic sequestration crisis and central
nervous system crisis were 62.3%, 24%, 10.3%,
2.7% and 0.7%, respectively. No priapism was observed
in any patient. No patients died and all were
discharged after treatment. Thirty-five painful
crisis episodes exhibited acute chest syndrome
in this study. Acute chest syndrome was recorded
based on the current criteria: new infiltrate
visible at chest X-ray associated with one or
more symptoms, such as fever, cough, tachypnea,
breathing difficulties or new-onset hypoxia.(12)
Blood exchange was performed in 2 of the patients
when no response to medical therapy was achieved.
Twenty-five of the 35 patients with acute chest
syndrome were in the severe pain group. VOC with
complications (acute chest syndrome, splenic sequestration
crisis, hepatic sequestration crisis and central
nervous system crisis) was more prevalent in the
group with severely painful crises. The prevalence
of complications rose with severity of pain. Nine
of the 15 patients with splenic sequestration
crises, all of the 4 patients with hepatic sequestration
crises and the one patient with central nervous
system crisis were in the severe pain group. Hemorrhagic
stroke was determined in our patients with central
nervous system crisis. Primary hemorrhagic stroke
is an uncommon complication of SCD, with reported
mortality rates of 24% to 65%. Most reported cases
are in adults, and little is known about the occurrence
in children. The incidence of hemorrhagic stroke
is greatly increased in patients with sickle cell
anemia (HbSS) compared with the general population
and affects children and young adults to a disproportionate
extent.(13) All 4 episodes with hepatic sequestration
were in the severe pain group. Acute hepatic sequestration
is a rarely recognized complication of VOC. Patients
with right upper quadrant hepatic syndrome generally
report right upper quadrant pain and fever. Clinical
examination is significant for jaundice and hepatic
enlargement.(14) Right upper quadrant pain and
fever, significant jaundice and hepatic enlargement
were present in all our cases. Blood exchange
transfusion was performed in all hepatic sequestration
attacks when no response was obtained to medical
therapy. Acute splenic sequestration crisis results
from the rapid sequestration of red blood cells
in the spleen. Splenic sequestration occurs in
10%-30% of children with SCD, most commonly between
the ages of 6 months and 3 years, and may follow
a febrile illness.(15,16) Similarly in this study,
splenic sequestration crisis was determined at
a level of 10.3%.
Infection is a major cause of morbidity and mortality
in these patients. Patients with SCD have impaired
immunity and are thus predisposed to infections
which frequently precipitate VOC. Infection was
also determined as the factor precipitating 82.2%
of painful crisis episodes in this study. Many
inflammatory markers of acute phase reaction are
elevated in SCD patients. Routine laboratory tests
including total leukocyte count and C-reactive
protein are sensitive for infection.(9,11) SCD
is considered an inflammatory condition due to
abnormally high leukocyte counts and increased
levels of WBCs during and after VOC. Clinical
studies show that leukocytosis is a risk factor
for major sickle cell-related complications such
as stroke, acute chest syndrome and early death.(17-19)
This study demonstrates that patients with SCD
have significantly high WBC levels in severely
painful episodes. As a marker of inflammation,
CRP has been used to predict prognosis and relapse
in patients with some chronic diseases, as well
as morbidity in others.(10) Akohoue et al.(10)
reported higher serum CRP levels in patients with
SCD than in healthy controls. In the present study,
CRP levels were higher in SCD patients with painful
crisis episodes. CRP levels were highest in patients
with severe crises. Previous studies have shown
that serum CRP levels are markedly increased in
patients with SCD with VOC and that sequential
measurements of CRP are useful in predicting the
subsequent development of severe painful crisis
in patients hospitalized for VOC.(20,21) Previous
studies have also shown that CRP levels correlate
well with VOC with fever in patients with SCD.(22)
Liver abnormality results in AST and ALT release,
making this a useful test for detecting liver
damage. Hemolysis also raises AST, ALT and bilirubin
levels in SCD.(23) One recently published study
reported elevated bilirubin, total protein random
glucose AST and ALT levels in SCD.(23) Ojuawo
et al.(24) reported significantly higher ALT,
alkaline phosphatase and bilirubin levels during
crisis than at recovery, especially in young patients.
However, total protein and albumin levels between
crisis and at recovery were not significantly
different. In this study, AST levels were significantly
high and albumin significantly low in the severe
pain crisis group. Koh et al.(25) reported that
increased hospital stay was associated with lower
albumin and hemoglobin/hematocrit levels. In agreement
with Koh et al.(25) we determined low albumin,
hemoglobin and hematocrit values in the severe
pain SCD group, that with the longest hospitalization.
This shows the presence of greater hemolysis and
hepatic damage in this group.
In conclusion, SCD continues to represent a major
public health problem in Turkey, and especially
in our region. Patients are mostly admitted to
the emergency department with VOC. This analysis
of 146 painful crisis episodes suggests that VDS
and FPS should be used in determining the severity
of painful episodes in patients with SCD, and
that patients with high pain intensity scores
should be monitored closely in terms of complicated
VOC.
Acknowledgement
We kindly thank our patients and their parents
for their contributions to this study.
1. Antmen B. Sickle cell anemia. Turk Arch Ped.
2009;44:39-42
2. Meremikwu MM, Okomo U. Sickle cell disease. Clin
Evid (Online) 2011;2011:2402
3. Ballas SK. Current issues in sickle cell pain
and its management. Hematology Am Soc Hematol Educ
Program. 2007:97-105
4. Portugal R, Morgado Loureiro M. Evaluation of
pain scale to predict hospital admission in patients
with sickle cell disease. Haematologica. 2003;88:ELT11
5. Guzeldem?r ME. Pain assessment methods. Sendrom.
1995:11-21
6. Jones KR, Vojir CP, Hutt E, Fink R. Determining
mild, moderate, and severe pain equivalency across
pain-intensity tools in nursing home residents.
J Rehabil Res Dev. 2007;44:305-314
7. Signorelli AA, Ribeiro SB, Moraes-Souza H, de
Oliveira LF, Ribeiro JB, da Silva SH, et al. Pain
measurement as part of primary healthcare of adult
patients with sickle cell disease. Rev Bras Hematol
Hemoter. 2013;35:272-277
8. Myrvik MP, Brandow AM, Drendel AL, Yan K, Hoffmann
RG, Panepinto JA. Clinically meaningful measurement
of pain in children with sickle cell disease. Pediatr
Blood Cancer. 2013;60:1689-1695
9. Patel DK, Mohapatra MK, Thomas AG, Patel S, Purohit
P. Procalcitonin as a biomarker of bacterial infection
in sickle cell vaso-occlusive crisis. Mediterr J
Hematol Infect Dis. 2014;6:e2014018. doi: 10.4084/MJHID.2014.018
10. Akohoue SA, Shankar S, Milne GL, Morrow J, Chen
KY, Ajayi WU, et al. Energy expenditure, inflammation,
and oxidative stress in steady-state adolescents
with sickle cell anemia. Pediatr Res. 2007;61:233-238
11. Ahmed SG. The role of infection in the pathogenesis
of vaso-occlusive crisis in patients with sickle
cell disease. Mediterr J Hematol Infect Dis. 2011;3:e2011028.
doi: 10.4084/MJHID.2011.028
12. Lionnet F, Hammoudi N, Stojanovic KS, Avellino
V, Grateau G, Girot R, et al. Hemoglobin sickle
cell disease complications: a clinical study of
179 cases. Haematologica. 2012;97:1136-1141
13. Strouse JJ, Hulbert ML, DeBaun MR, Jordan LC,
Casella JF. Primary hemorrhagic stroke in children
with sickle cell disease is associated with recent
transfusion and use of corticosteroids. Pediatrics.
2006;118:1916-1924
14. Norris WE. Acute hepatic sequestration in sickle
cell disease. J Natl Med Assoc. 2004;96:1235-1239
15. Bender MA, Hobbs W. Sickle Cell Disease. In:
Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR,
Fong CT, Smith RJH, Stephens K, (eds). GeneReviews®
[Internet]. Seattle (WA), University of Washington,
Seattle; 1993-2014.
2003 Sep 15 [updated 2012 May 17].
16. Al-Salem AH. Splenic complications of sickle
cell anemia and the role of splenectomy. ISRN Hematol.
2011;2011:864257. doi: 10.5402/2011/864257
17. Chies JA, Nardi NB. Sickle cell disease: a chronic
inflammatory condition. Med Hypotheses. 2001;57:46-50
18. Goncalves MS, Queiroz IL, Cardoso SA, Zanetti
A, Strapazoni AC, Adorno E, et al. Interleukin 8
as a vaso-occlusive marker in Brazilian patients
with sickle cell disease. Braz J Med Biol Res. 2001;34:1309-1313
19. Schimmel M, Nur E, Biemond BJ, van Mierlo GJ,
Solati S, Brandjes DP, et al. Nucleosomes and neutrophil
activation in sickle cell disease painful crisis.
Haematologica. 2013;98:1797-803
20. Bargoma EM, Mitsuyoshi JK, Larkin SK, Styles
LA, Kuypers FA, Test ST. Serum C-reactive protein
parallels secretory phospholipase A2 in sickle cell
disease patients with vasoocclusive crisis or acute
chest syndrome. Blood. 2005;105:3384-3385
21. Hedo CC, Aken'ova YA, Okpala IE, Durojaiye AO,
Salimonu LS. Acute phase reactants and severity
of homozygous sickle cell disease. J Intern Med.
1993;233:467-470
22. Unal S, Arslankoylu AE, Kuyucu N, Aslan G, Erdogan
S. Procalcitonin is more useful than C-reactive
protein in differentiation of fever in patients
with sickle cell disease. J Pediatr Hematol Oncol.
2012;34:85-89
23. Pandey S, Sharma A, Dahia S, Shah V, Sharma
V, Mishra RM, et al. Biochemical indicator of sickle
cell disease: preliminary report from India. Indian
J Clin Biochem. 2012;27:191-195
24. Ojuawo A, Adedoyin MA, Fagbule D. Hepatic function
tests in children with sickle cell anaemia during
vaso occlusive crisis. Cent Afr J Med. 1994;40:342-345
25. Koh C, Turner T, Zhao X, Minniti CP, Feld JJ,
Simpson J, et al. Liver stiffness increases acutely
during sickle cell vaso-occlusive crisis. Am J Hematol.
2013;88:E250-4. doi: 10.1002/ajh.23532
|
