|
A Clinicopathologic
study of Lichen Planus at Assir area, Kingdom
of Saudi Arabia
......................................................................................................................................................................
Hamad Al-Fahaad
Correspondence:
Hamad Al-Fahaad
Department of Dermatology
Assir Central Hospital
Abha
Saudi Arabia
Telephone: (966) 500-544495
Email: hamadyam@gmail.com
|
ABSTRACT
Background: Lichen planus (LP) is
a subacute or chronic immunologically mediated
dermatosis that involves the skin, mucous
membranes, hair follicles, and nails. To
date, the clinicopathologic features of
these lesions in Assir region, Kingdom of
Saudi Arabia are largely unknown.
Materials and Methods: To define
these features, diagnostic records of dermatopathology
cases received at the Pathology Department,
Assir Central Hospitals (2007-2008 ) were
reviewed. The lesions included 51 cases
of lichen planus.
Results: Lichen planus was more common
in males than in females (2 : 1). The average
age incidence was 35.8 3.2 years, and 35.4
2.4 years for males and females respectively.
The lower extremities, face and trunk were
the most common sites for the lichen planus.
Conclusions: In Assir region, Kingdom
of Saudi Arabia lichen planus is a common
disease. It usually affects middle age populations
and has a male sex predilection.
Key words: Lichen planus, Saudi Arabia
|
Normal skin
The epidermis is a stratified squamous epithelium
composed predominantly of keratinocytes, and of
at least three other resident cells: melanocytes,
Langerhans cells and Merkel cells.
The keratinocytes differ from the dendritic cells,
or clear T-cells, by having intercellular bridges
and ample amount of stainable cytoplasm. The epidermis
is not only the most cellular but also the most
dynamic layer of the skin. As such, it continuously
sheds and regenerates itself.
The keratinocytes are arranged in four layers:
the basal cell layer (stratum basalis), the squamous
cell layer (stratum spinosum), the granular layer
(stratum granulosum) and the horny layer (stratum
corneum).(18)
The epidermis forms a broadly undulating interface
with the dermis. It extends into the dermis as
broad folds (the rete ridges) and the dermis projects
into the epidermis in finger-like projections
(the dermal papillae). (18)
The basal cell layer is formed of a single layer
of columnar cells. They lie with their long axes
perpendicular to the dividing line between the
epidermis and the dermis. They have a more basophilic
cytoplasm than cells of the stratum spinosum and
contain dark stained oval or elongated nuclei.
They often contain melanin pigment transferred
from adjacent melanocytes. The extent and distribution
of this pigment correlates with skin color. The
basal cells are connected with each other and
with the overlying cells by intercellular bridges
or desmosomes. At their base, they are attached
to the subepidermal BM zone by modified desmosomes;
termed hemidesmosomes. The basal cells and the
overlying squamous cells contain keratin intermediate
filaments termed tonofilaments, which form the
developing cytoskeleton. Most of the mitotic activity
in normal epidermis occurs in the basal cell layer.(1
and 18)
The second layer is the prickle cell layer. It
is formed of 5 to 10 layers of polyhedral cells
that become flattened toward the surface. The
cells are separated by spaces that are transversed
by intercellular bridges. The tonofilaments within
the cytoplasm of these cells are loose bundles
of electron-dense filaments. They are attached
to the attachment plaque of a desmosome at one
end, and the other end lies free in the cytoplasm
near the nucleus. The intercellular cement substance
between two adjacent keratinocytes contains glycoproteins.
It has a gel-like consistency that explains why
it on one hand provides cohesion between the epidermal
cells and on the other hand allows the rapid passage
of water-soluble substances through the intercellular
spaces. Furthermore, it allows the opening up
of desmosomes and individual cell movement. (22)
Recent studies established the molecular basis
for cell-to-cell adhesion within the prickle cell
layer and other epidermal layers. Cadherins is
a key family of adhesion molecules that are derived
from multiple genes. Desmosomal cadherins are
desmogleins and desmocollins that localize to
desmosomes. They are linked to intracytoplasmic
intermediate filaments by plakoglobin and desmoplakin.
(22)
The third layer is the granular cell layer. It
is composed of flattened cells and their cytoplasm
is filled with keratohyaline granules that are
deeply basophilic. The thickness of the granular
layer in normal skin is proportional to the thickness
of the horny layer. It is only 1-3 cell layers
thick in areas with a thin horny layer. It reaches
up to 10 layers in areas with a thick horny layer
such as the palm and sole. There is often an inverse
relationship between the presence and thickness
of the granular cell layer and parakeratosis.
For instance in psoriasis, parakeratosis is associated
with markedly attenuated or absent granular cell
layer. The keratohyaline granules are the precursors
of the protein filaggrin that promotes aggregation
of keratin filaments in the cornified layer. (15)
The fourth layer is the horny layer. It is composed
of multiple layers of polyhedral cells that are
arranged in a basket weave pattern. These cells
lose their nucleus and cytoplasmic organelles
and are composed entirely of keratin filaments.
These cells are the most differentiated cells
of the keratinizing system. They eventually shed
from the surface of the skin. (17) The basement
membrane separates the epidermal basal layer from
the dermis. It is seen by light microscopy, as
a continuous and thin periodic acid Schiff (PAS)-stained
layer. Alternatively, by electron microscopy,
the basal cells are seen attached to the basal
lamina by hemidesmosomes. (15)
Ultrastructurally, the basal lamina is composed
of four different regions. From the epidermis
to the dermis, they are respectively: i) the plasma
membrane of the basal cells containing the hemidesmosomes
and anchoring filaments (15), ii) the lamina Lucida
which represents an electron-lucent area composed
of laminin and bullous pemphigoid antigen, iii)
the lamina densa, an electron-dense area composed
of type IV collagen, and iv) the sublamina densa
or lamina fibroreticularis containing the structures
that attach the basal lamina to the connective
tissue of the dermis. The latter represents extension
of the lamina densa, the anchoring fibrils (type
VII collagen) and the antigen to epidermolysis
bullosa acquista. (22)
The supportive structure of the skin is provided
by the dermis, a relatively hypocellular layer
of varying thickness. It is composed of a structural
collagen matrix, elastin and ground substance.
Embedded within the dermis are epidermal appendages,
nerve endings, resident cells and vessels. The
dermis is divided into two compartments; the papillary
dermis and the reticular dermis. The papillary
dermis underlies the epidermis and extends around
the adenexa (in which location it is also known
as the adventitial dermis). It is composed of
fine fibers consisting predominantly of type I
and type III collagen. It moors the epidermis,
interdigitates with the reticular dermis, and
surrounds the epidermal appendages. It also contains
a delicate branching network of fine elastic fibers,
abundant ground substance, superficial capillary
plexuses and fibroblasts. (21)
The reticular dermis is thicker than the papillary
dermis. It is made up of densely packed coarse-fibered
collagen which is predominantly type I. The collagen
bundles traverse the dermis in a pattern that
has not yet been defined. Associated with these
two interstitial collagens are the finely filamentous
collagens type V and type VI. (9) Supplementing
its protective function, the skin has three specialized
redundancies referred to as epidermal appendages
or adnexa. These epidermis-derived structures
consist of the pilosebaceous apparatus (with its
hair, sebaceous and apocrine elements), the eccrine
glands and the nails.
Lichen planus
Lichen planus (LP) is a subacute or chronic immunologically
mediated dermatosis that involves the skin, mucous
membranes, hair follicles, and nails. (6)
Pathophysiology: Although the exact cause
of lichen planus is unknown, a cell-mediated immune
reaction has been implicated in its pathogenesis.
In support, lichen planus is associated with other
diseases of altered immunity, such as ulcerative
colitis, alopecia areata, vitiligo, dermatomyositis,
morphea, lichen sclerosus and myasthenia gravis.
In addition an association is noted among lichen
planus and hepatitis C infection, chronic active
hepatitis and primary biliary cirrhosis. (23,24
and 25)
Immunohistochemical studies show that the infiltrating
cells in lichen planus are predominantly T lymphocytes
with very few B lymphocytes. The predominant subtypes
of T lymphocytes in the infiltrate are of helper-inducer
or suppressor-cytotoxic T lymphocytes lineage.
Both subsets participate in the immunologic reaction
with the suppressor-cytotoxic T lymphocytes being
predominant in the epidermotropic response, suggesting
a cell-mediated cytotoxic mechanism against the
epidermal cells. The basal keratinocytes adjacent
to the infiltrate express intercellular adhesion
molecule-1, which enhances the interaction between
lymphocytes and their epidermal targets, resulting
in keratinocytic destruction.( 34)
This surface antigen is probably induced by cytokines
released by lymphocytes from the infiltrate. In
addition, a superantigen may be involved in the
pathogenesis of lichen planus. (2,3,5,6,7,8,9,11
and 26)
The number of Langerhans' cells in the epidermis
is increased very early in the disease. Immunoelectron
studies have shown close contacts of lymphocytes
with Langerhans' cells and macrophages. (14) The
Langerhans' cells can process and present antigens
to T lymphocytes, leading to their stimulation
and thus attacking keratinocytes. These cell-to-cell
interactions suggest that a cell mediated immune
mechanism is operative in lichen planus. (31 and
32)
Incidence: Lichen planus has a worldwide
distribution with no significant geographical
variation in its incidence. It can occur at any
age, with a tendency to affect middle aged and
elderly individuals. No sex predilection has been
noted. (6 and 7)
Clinical features: The eruption of lichen
planus is characterized by small, flat-topped,
shiny, violaceous papules that may coalesce into
plaques. The papules are polygonal and often show
a network of white lines known as Wickham's striae.
They vary in size from 1 mm to greater than 1
cm. The disease has a predilection for the flexor
surfaces of the forearms and legs. Pruritus is
very common, but it varies in severity according
to the type of lesion and extent of involvement.
Hypertrophic lesions are usually extremely pruritic.
The eruption of lichen planus may be localized
or generalized, and Koebner's phenomenon is commonly
seen. (6 and 7)
In addition to the cutaneous eruption, lichen
planus may involve the mucous membranes of the
buccal mucosa and tongue (oral LP), genitalia,
nails, and scalp.
The oral lesions of lichen planus are common and
may occur as a sole manifestation of the disease
or be associated with cutaneous involvement. They
usually involve the buccal and glossal mucosa
in the form of a reticular network of coalescent
papules. Besides this reticular type, other lesional
patterns have been described in oral lichen planus,
such as papular, plaquelike, atrophic, erosive,
and bullous. (6 ,28and 30) Genital involvement
is common in men with cutaneous lichen planus,
usually in the form of an annular configuration
of papules on the glans penis. Less commonly,
linear white striae may be seen. The nails are
involved in about 10% of cases of lichen planus,
in the form of roughening, longitudinal ridging,
and, rarely, thinning and destruction. (16,29
and 31)
Lichen planopilaris is a type of lichen planus
that predominantly affects the scalp with follicular
and perifollicular violaceous scaly pruritic papules.
It may coexist with typical lichen planus lesions
on the skin, mucous membranes, or nails. Progressive
hair loss may occur, resulting in the development
of irregularly shaped atrophic patches of scarring
alopecia on the scalp (pseudopelade of Brocq).
The axillae and the pubic region may also be affected.
Hyperkeratotic follicular papules may also be
seen on glabrous skin. The Graham Little syndrome
consists of an association of scarring alopecia
of hair-bearing areas and hyperkeratotic papules
on glabrous skin. Linear lichen planopilaris of
the face resolving with scarring has also been
described. (17)
Other variants of lichen planus include hypertrophic
lichen planus, atrophic lichen planus, vesicular
lichen planus, lichen planus pemphigoides, ulcerative
lichen planus, actinic lichen planus, annular
lichen planus, linear lichen planus, and guttate
lichen planus. The hypertrophic lichen planus
is a common variant of lichen planus that usually
affects the extensor surfaces of the lower extremities,
especially around the ankles. It is a pruritic
lesion that consists of thickened, often verrucous
plaques that may heal with residual pigmentation
and scarring. The atrophic variant of lichen planus
is characterized by a few lesions that are often
the resolution of annular or hypertrophic lesions.
The vesicular lichen planus is a rare variant
that shows vesicles situated on some of the preexisting
lichen planus lesions.
The lichen planus pemphigoides differs from vesicular
lichen planus by its more disseminated eruption
and more extensive bullae. In addition, lichen
planus pemphigoides may arise from papules of
lichen planus and normal-appearing skin.
The ulcerative lichen planus is a rare variant
of lichen planus, which shows bullae, erosions,
and painful ulcerations on the feet and toes resulting
in atrophic scarring and permanent loss of the
toenails. It is usually associated with cutaneous
and oral lesions of lichen planus, as well as
atrophic alopecia of the scalp. The actinic lichen
planus (lichen planus actinicus or pigmentosus)
usually develops in spring and summer on sun-exposed
areas of the skin, particularly the face. It is
characterized by annular plaques with central
blue to light brown pigmentation and well-defined,
slightly raised, hypopigmented borders. Pruritus
is minimal or absent.
Three forms of actinic lichen planus have been
described: annular, pigmented, and dyschromic.
(27 and 32)
The annular lichen planus is characterized by
annular lesions with an atrophic center usually
found on the buccal mucosa and male genitalia.
Lichen planus papules that are purely annular
are rare. The linear lichen planus represents
a zosteriform lesion or may develop as a Koebner's
effect. Finally, the guttate lichen planus develops
in the form of discrete lesions which may vary
in size from 1 mm to 1 cm. They almost never become
chronic. Of note, the hypertrophic and actinic
variants of lichen planus are commoner than the
other variants. (6)
Histopathologic features: The typical papules
of lichen planus show the following histologic
features: 1) compact orthokeratosis, which contains
very few, if any, parakeratotic cells, 2) wedge-shaped
hypergranulosis with the granular cells being
increased in number and size, and contain more
abundant coarse keratohyaline granules, 3) irregular
acanthosis, which affects the spinous layer of
the rete ridges as well as the suprapapillary
plates. The rete ridges show irregular lengthening,
and some of them are pointed at their lower end,
giving them a saw-toothed appearance, 4) destruction
of the basal cell layer, which is obvious in fully
developed lesions. In these lesions, the basal
layer has the appearance of flattened squamous
cells (squamatization of the basal layer), and
5) a band-like (lichenoid) dermal inflammatory
infiltrate, which is in close approximation to
the epidermis and is sharply demarcated at its
lower border. It is composed mainly of lymphocytes
intermingled with macrophages. Melanophages are
usually seen in the papillary and upper reticular
dermis as a result of destruction of the basal
cells with subsequent pigment incontinence. (4)
In addition, apoptotic keratinocytes are present
in the lower epidermis and papillary dermis in
most cases. They appear in the form of round or
oval, homogenous, eosinophilic bodies (colloid,
hyaline, or Civatte bodies). Occasionally, small
areas of artifactual separation between the epidermis
and the dermis are present and are known as Max-Joseph
spaces. In some instances, this separation occurs
in vivo as a result of extensive damage to the
basal cells. Wickham's striae are caused by the
focal increase in the thickness of the granular
layer and of the total epidermis. (20)
The typical papules of lichen planus show the
following histologic features: 1) compact orthokeratosis,
which contains very few, if any, parakeratotic
cells, 2) wedge-shaped hypergranulosis with the
granular cells being increased in number and size,
and contain more abundant coarse keratohyaline
granules, 3) irregular acanthosis, which affects
the spinous layer of the rete ridges as well as
the suprapapillary plates. In addition, variants
of lichen planus have additional histological
changes. In this regard, the hypertrophic lichen
planus shows considerable acanthosis, papillomatosis,
and hyperkeratosis. The vesicular lichen planus
usually shows large Max-Joseph spaces, with subepidermal
blisters. The lichen planus pemphigoides that
arises from uninvolved skin shows subepidermal
bullae with an inflammatory infiltrate that is
not band-like and contains eosinophils. The lichen
planus actinicus may show histologic features
similar to those of typical lichen planus, but
with a tendency toward thinning of the epidermis
in the center of the lesion. In addition, more
evident pigment incontinence and numerous melanophages
are usually present in the papillary and upper
reticular dermis. The oral lichen planus may show
parakeratosis rather that orthokeratosis, with
the presence of a granular layer (the buccal mucosa
is normally devoid of a granular layer, except
in the hard palate). The epithelium is often atrophic,
and ulcerations may develop. The lichen planopilaris
usually shows a focally dense, band-like perifollicular
lymphocytic infiltrate. Vacuolar changes of the
basal layer of the outer root sheath and necrotic
keratinocytes are often seen. Advanced cases may
show perifollicular fibrosis and epithelial atrophy,
which may result in scarring alopecia. (10,19
and 33)
In this investigation, we took
an aim at studying the clinicopathologic features
of lichen planus in Assir region, Kingdom of Saudi
Arabia. To explore this aim and to fill this existing
gap in literature, we carried out this investigation.
To achieve our goals, we examined clinical and
pathological characteristics of these lesions.
A total of 51 lesions representing lichen planus
were examined.
Tissue specimens: The
formalin fixed, paraffin embedded tissues were
obtained from the Department of Pathology, in
Assir region, Kingdom of Saudi Arabia. The total
number of specimens was 51 cases, including 51
cases of lichen planus. Clinical data were obtained
from the clinical referral reports. They included:
age and sex of the patient, type of lesions, and
the site, and number of these lesions. All the
patients were Saudi (Caucasian). No black individuals
were included in this study.
Clinical features of lichen
planus: The study group consisted of 51 patients,
including 17 females and 34 males. Evaluation
of the clinical and histological profiles of the
lesions in our locality (Assir region) demonstrated
that they usually tend to affect the middle age
groups and had male sex predilection.
Clinical features: The study group consisted
of 51 patients, including 34 males (34/51, 67%)
and 17 females (17/51, 33%). The clinical data
were obtained from the referral clinical reports.
The clinical characteristics of these lesions
were summarized in Table 1-2 and Figure 2.
Table 1: Clinical characteristics of lichen
planus in males
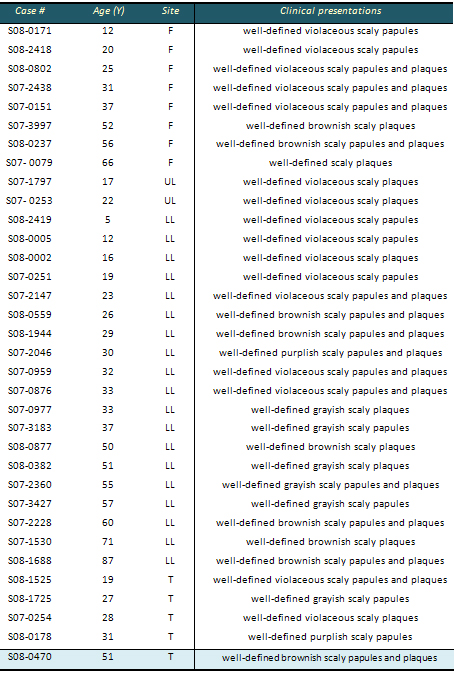
F : Face
LL : Lower Limb
UL : Upper Limb
T : Trunk
Table 2: Clinical characteristics of lichen
planus in females
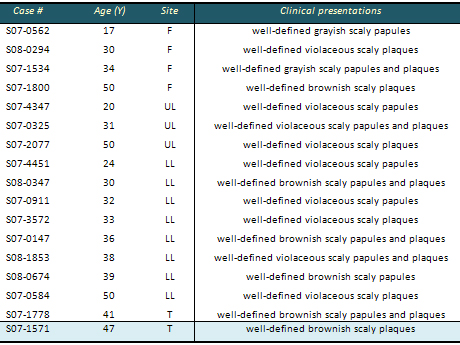
F : Face
LL : Lower Limb
UL : Upper Limb
T : Trunk
Figure 1: The normal skin is composed of three
layers: 1) epidermis, 2) dermis, 3) the subcutaneous
adipose tissue. Each layer has a complex structure
and function. The keratinocytes of the epidermis
are arranged in four layers: 1) stratum germinatum,
2) stratum spinosum, 3) stratum granulosum, and
4) stratum corneum
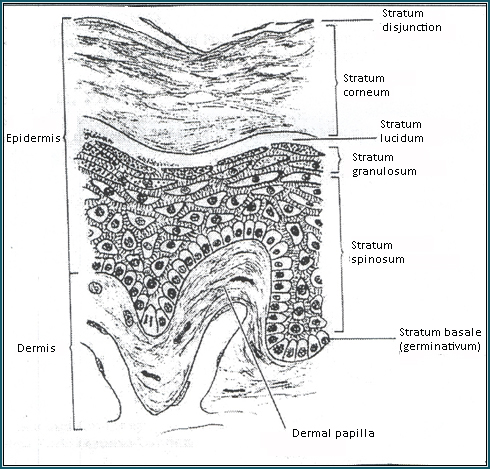
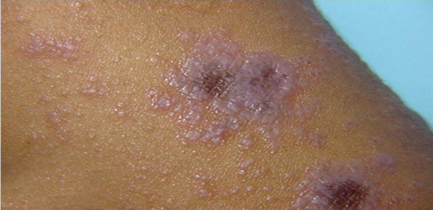
Figure 2: Lichen planus, violaceous flat-topped
scaly papules and plaques
Pathological features: The typical papules
of lichen planus show the following histologic
features: 1) compact orthokeratosis, which contains
very few, if any, parakeratotic cells, 2) wedge-shaped
hypergranulosis with the granular cells being
increased in number and size, and contain more
abundant coarse keratohyaline granules, 3) irregular
acanthosis, which affects the spinous layer of
the rete ridges as well as the suprapapillary
plates. The rete ridges show irregular lengthening,
and some of them are pointed at their lower end,
giving them a saw-toothed appearance, 4) destruction
of the basal cell layer, which is obvious in fully
developed lesions. In these lesions, the basal
layer has the appearance of flattened squamous
cells (squamatization of the basal layer), and
5) a band-like (lichenoid) dermal inflammatory
infiltrate, which is in close approximation to
the epidermis and is sharply demarcated at its
lower border. It is composed mainly of lymphocytes
intermingled with macrophages. Melanophages are
usually seen in the papillary and upper reticular
dermis as a result of destruction of the basal
cells with subsequent pigment incontinence (Figure
3).
Figure 3 : Histological features of lichen
planus. dense dermal inflammatory infiltrate.
Occasional inflammatory cells are seen abutting
on the basal cell keratinocytes together with
apoptotic keratinocytes
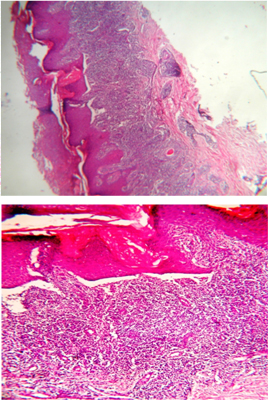
In addition, apoptotic keratinocytes are present
in the lower epidermis and papillary dermis in
most cases. They appear in the form of round or
oval, homogenous, eosinophilic bodies (colloid,
hyaline, or Civatte bodies). Occasionally, small
areas of artifactual separation between the epidermis
and the dermis are present and are known as Max-Joseph
spaces. In some instances, this separation occurs
in vivo as a result of extensive damage to the
basal cells (Figure 3).
|
DISCUSSION AND CONCLUSIONS |
Interface dermatitis encompasses
a wide range of lesions characterized by lichenoid
and vacuolar changes at the dermoepidermal junction
i.e. interface zone. The former is characterized
by lichenoid infiltrate and basal cell keratinocytes
damage (LID). In the vacuolar subtype (VID), vacuolation
of the basal cell keratinocytes is a characteristic
feature. Although these lesions are thought to
be autoimmune in nature, their exact pathogenetic
causes are still unknown. In this vein, ID lesions
are multifactorial in origin; may be induced by
drugs; or by complex environmental, genetic and
life style factors. (28)
Interestingly, a new association between these
lesions and chronic liver disease has emerged.
In this respect, Harman and his colleagues found
a close association between LP and hepatitis C
infection. (13) Also, anti hepatitis C antibodies
were seen in patients with lichen planus. (13)
The clinical features of ID lesions in our series
(age incidence, female sex predilection, and site
of affection and the average duration of the diseases)
are comparable to the findings in western societies.
The studies performed in these societies reported
an average male/female ratio of 1:1 to 1:1.3.
The mean age was about 50.4 years. (6) Taken together,
these findings suggest common underlying pathogenetic
mechanisms in these diseases.
The female sex predilection may indicate that
the susceptible genotype is probably characterized
by a single inherited dominant allele on the X-chromosome.
The disease chronicity, adult onset, female sex
predilection and association with other autoimmune
diseases suggest the autoimmune nature of ID.
Familial lichen planus was reported by others
in several studies and this raised the suggestion
of genetic predisposition in ID. Several studies
examined the role of genetic factors on the development
of ID lesions like lichen planus such as HLA-associated
antigens.
They showed a role of these antigens in the recruitment
of lymphocytes at the site of inflammation.
The diagnosis of lichen planus can be usually
made on histologic grounds in more than 90% of
cases. However, a number of diseases may simulate
the histologic picture of lichen planus and make
some difficulties in the diagnosis. These lesions
include LP-like keratosis, lichenoid drug eruption,
lichenoid lupus erythematosus, chronic graft-versus-host
disease, and lichen simplex chronicus. Lichen
planus -like keratosis shows focal parakeratosis
and adjacent solar lentigines in an otherwise
typical histological picture of lichen planus.
Lichenoid drug eruptions can be differentiated
from lichen planus by the presence of focal parakeratosis
with concomitant agranulosis, exocytosis of lymphocytes
within the epidermis, and numerous eosinophils
in a deeper inflammatory infiltrate. Lichenoid
lupus erythematosus differs from lichen planus
in the presence of epidermal atrophy in addition
to acanthosis, perivascular and periadnexal infiltrate
in addition to the superficial band-like infiltrate,
dermal mucin deposits, and the presence of a thickened
PAS-positive basement membrane. Chronic graft-versus-host
disease may show epidermal changes similar to
lichen planus. However, the inflammatory infiltrate
tends to be perivascular and the number of Langerhans'
cells is decreased in chronic graft-versus-host
disease. Lichen simplex chronicus can be differentiated
from lichen planus by the absence of both basal
cell destruction and the band-like infiltrate.
(35)
The prognosis for lichen planus is good, as most
cases regress within 18 months. However, some
cases may recur. Hypertrophic lesions may leave
residual hyperpigmentation. Alopecia is often
permanent. Malignant transformation of cutaneous
LP occurs in less than 1% of cases. (36) Squamous
cell carcinoma may arise occasionally in long-standing
lesions of lichen planus situated on mucous membranes
or the vermillion border . (12) Ulcerative lesions
of oral lichen planus, particularly in men, have
a higher risk for malignant transformation. (37)
1- Affolter VK, Moore PF (1994) : Histologic features
of normal canine and feline skin. Clin Dermatol;12:491-7.
2- Akasu R, From L, Kahn HJ (1993) : Lymphocyte
and macrophage subsets in active and inactive lesions
of lichen planus. Am J Dermatopathol;15:217-23.
3- al-Fouzan AS, Habib MA, Sallam TH, el-Samahy
MH, Rostom AI (1996): Detection of T lymphocytes
and T lymphocyte subsets in lichen planus: in situ
and in peripheral blood. Int J Dermatol;35:426-9.
4- Balasubramaniam P, Ogboli M, Moss C (2008): Lichen
planus in children: review of 26 cases. Clin Exp
Dermatol;33:457-9.
5- Bhan AK, Harrist TJ, Murphy GF, Mihm MC, Jr.(
1981): T cell subsets and Langerhans cells in lichen
planus: in situ characterization using monoclonal
antibodies. Br J Dermatol;105:617-22.
6- Bhattacharya M, Kaur I, Kumar B: Lichen planus
(2000): a clinical and epidemiological study. J
Dermatol;27:576-82.
7- Bjerke JR, Matre R (1983): Demonstration of Ia-like
antigens on T lymphocytes in lesions of psoriasis,
lichen planus and discoid lupus erythematosus. Acta
Derm Venereol;63:103-7.
8- Bramanti TE, Dekker NP, Lozada-Nur F, Sauk JJ,
Regezi JA (1995): Heat shock (stress) proteins and
gamma delta T lymphocytes in oral lichen planus.
Oral Surg Oral Med Oral Pathol Oral Radiol Endod;80:698-704.
9- Bruckner-Tuderman L (1994): Collagen VII and
bullous disorders of the skin. Dermatology;189 Suppl
2:16-20.
10- Bruno E, Alessandrini M, Russo S, D'Erme G,
Nucci R, Calabretta F (2002): Malignant degeneration
of oral lichen planus: our clinical experience and
review of the literature. An Otorrinolaringol Ibero
Am;29:349-57.
11- Buechner SA (1984): T cell subsets and macrophages
in lichen planus. In situ identification using monoclonal
antibodies and histochemical techniques. Dermatologica;169:325-9.
12- Katz RW, Brahim JS, Travis WD (1990): Oral squamous
cell carcinoma arising in a patient with long-standing
lichen planus. Oral Surg Oral Med Oral Pathol 70:282-285
13- Chainani-Wu N, Lozada-Nur F, Terrault N (2004):
Hepatitis C virus and lichen planus: a review. Oral
Surg Oral Med Oral Pathol Oral Radiol Endod;98:171-83.
14- Fayyazi A, Schweyer S, Soruri A, Duong LQ, Radzun
HJ, Peters J, Parwaresch R, Berger H (1999): T lymphocytes
and altered keratinocytes express interferon-gamma
and interleukin 6 in lichen planus. Arch Dermatol
Res;291:485-90.
15- Frenk E, Benathan M (1992): Current concepts
in pediatric burn care: morphology and biology of
normal human skin and stratified cultures of epidermal
cells for wound covering. Eur J Pediatr Surg;2:207-9.
16- Groves RW, Rauschmayr T, Nakamura K, Sarkar
S, Williams IR, Kupper TS (1996): Inflammatory and
hyperproliferative skin disease in mice that express
elevated levels of the IL-1 receptor (type I) on
epidermal keratinocytes. Evidence that IL-1-inducible
secondary cytokines produced by keratinocytes in
vivo can cause skin disease. J Clin Invest;98:336-44.
17- Kanitakis J (1998) : Immunohistochemistry of
normal human skin. Eur J Dermatol;8:539-47.
18- Kanitakis J (2002): Anatomy, histology and immunohistochemistry
of normal human skin. Eur J Dermatol;12:390-9; quiz
400-1.
19- Lanfranchi-Tizeira HE, Aguas SC, Sano SM (2003):
Malignant transformation of atypical oral lichen
planus: a review of 32 cases. Med Oral;8:2-9.
20- Luis-Montoya P, Dominguez-Soto L, Vega-Memije
E (2005): Lichen planus in 24 children with review
of the literature. Pediatr Dermatol;22:295-8.
21- Marples MJ (1969): The normal flora of the human
skin. Br J Dermatol;81:Suppl 1:2-13.
22- Nazzaro V (1989): [Normal development of human
fetal skin]. G Ital Dermatol Venereol;124:421-7.
23- Ognjenovic M, Karelovic D, Cekic-Arambasin A,
Tadin I, Vrebalov-Cindro V (1998a) : Oral lichen
planus and HLA DR. Coll Antropol;22 Suppl:97-101.
24- Ognjenovic M, Karelovic D, Cindro VV, Tadin
I (1998b): Oral lichen planus and HLA A. Coll Antropol
;22 Suppl:89-92.
25- Ognjenovic M, Karelovic D, Mikelic M, Tadin
I, Vrebalov-Cindro V (1998c) : Oral lichen planus
and HLA B. Coll Antropol ;22 Suppl:93-96.
26- Popp JW, Jr., Harrist TJ, Dienstag JL, Bhan
AK, Wands JR, LaMont JT, Mihm MC, Jr. (1981): Cutaneous
vasculitis associated with acute and chronic hepatitis.
Arch Intern Med;141:623-9.
27- Ragaz A, Ackerman AB (1981): Evolution, maturation,
and regression of lesions of lichen planus. New
observations and correlations of clinical and histologic
findings. Am J Dermatopathol;3:5-25.
28- Scully C, Beyli M, Ferreiro MC, Ficarra G, Gill
Y, Griffiths M, Holmstrup P, Mutlu S, Porter S,
Wray D (1998): Update on oral lichen planus: etiopathogenesis
and management. Crit Rev Oral Biol Med;9:86-122.
29- Segaert S, Tabernero J, Chosidow O, Dirschka
T, Elsner J, Mancini L, Maughan T, Morere JF, Santoro
A, Sobrero A, Van Cutsem E, Layton A (2005): The
management of skin reactions in cancer patients
receiving epidermal growth factor receptor targeted
therapies. J Dtsch Dermatol Ges;3:599-606.
30- Seoane J, Romero MA, Varela-Centelles P, Diz-Dios
P, Garcia-Pola MJ (2004): Oral lichen planus: a
clinical and morphometric study of oral lesions
in relation to clinical presentation. Braz Dent
J;15:9-12.
31- van den Akker TW (2001): [Lichen planus, a T-lymphocyte
mediated reaction involving the skin and mucous
membranes]. Ned Tijdschr Geneeskd;145:1921-8.
32- van den Akker TW, Reker CH, Knol A, Post J,
Wilbrink J, van der Veen JP (2001): Teledermatology
as a tool for communication between general practitioners
and dermatologists. J Telemed Telecare;7:193-8.
33- Yaacob HB, Tan PL, Ngeow WC (2002): Malignancy
in oral lichen planus: a review of a group from
the Malaysian population. J Oral Sci;44:65-71.
34- Bennion, M.H. Middleton, K.M. David-Bajar, S.
Brice and D.A. Norris (1995) : In three types of
interface dermatitis, different patterns of expression
of intercellular adhesion molecule-1 (ICAM-1) indicate
different triggers of disease, J Invest Dermatol
105
35- Prieto VG, Casal M, McNutt NS (1993) : Lichen
planus like keratosis Am J Surg Pathol
36- Sigurgeirsson B, Lindelof B. (1991) : Lichen
planus and malignancy. An epidemiologic study of
2071 patients and a review of the literature. Arch
Dermatol
37- Ogmundsdottir, H. Hilmarsdottir, A. Astvaldsdottir,
J.H. Johansson (2002): A study of oral mucosa in
Iceland, Eur J Oral Sci 110
|
